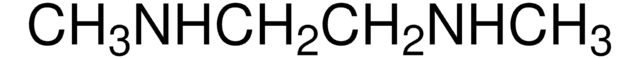39380
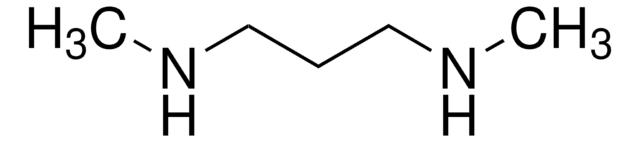
3-(Dimethylamino)-1-propylamine
purum, ≥98.0% (GC)
Synonym(s):
N,N-Dimethyl-1,3-diaminopropane, N,N-Dimethyl-1,3-propanediamine
About This Item
Recommended Products
vapor density
3.6 (vs air)
Quality Level
vapor pressure
5 mmHg ( 20 °C)
grade
purum
assay
≥98.0% (GC)
form
liquid
impurities
≤2% water
refractive index
n20/D 1.435 (lit.)
n20/D 1.436
bp
133 °C (lit.)
density
0.812 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CN(C)CCCN
InChI
1S/C5H14N2/c1-7(2)5-3-4-6/h3-6H2,1-2H3
InChI key
IUNMPGNGSSIWFP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a cationic moiety to form the ionic liquid, 3-(dimethylamino)-1-propylaminium formate ([DMAPA]FA) which is a potential reagent to extract protein from yeast cells.
- As an aminating agent in the palladium-catalyzed amination of aryl amines.
- In the derivatization of pullulan to form cationic pullulan.
- In the partial modification of poly(styrene-alt-maleic anhydride) to form N-substituted maleimide.
- As a test compound in the parallel synthesis of alkylamine lipidoids.
- To cleave the polyamides synthesized by solid phase methods on Boc-β-Ala-PAM resin.
- Synthesis of (N,N-dialkylamino)alkyl-2-amino benzamide by reacting with isatoic anhydride.
- As a reagent for the anomeric deacetylation of protected sugars.
- Preparation of bioreducible copolymers used in gene delivery.
Other Notes
signalword
Danger
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - Skin Sens. 1 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
89.6 °F - closed cup
flash_point_c
32 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service