70082
Myristic acid
≥98.0% (GC)
Synonym(s):
1-Tridecanecarboxylic acid, C14:0, NSC 5028, Tetradecanoic acid
About This Item
Recommended Products
Quality Level
assay
≥98.0% (GC)
form
powder
bp
250 °C/100 mmHg (lit.)
mp
52-54 °C (lit.)
53-56 °C
functional group
carboxylic acid
lipid type
saturated FAs
shipped in
ambient
Storage temp.
room temp
SMILES string
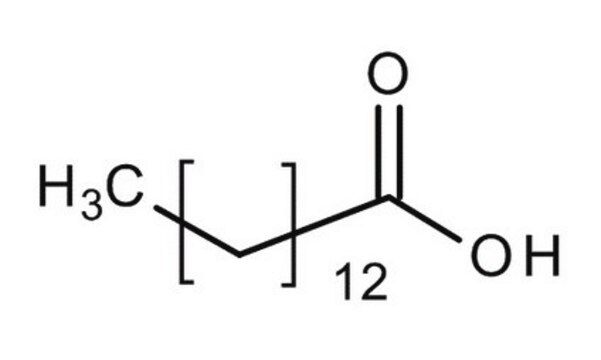
CCCCCCCCCCCCCC(O)=O
InChI
1S/C14H28O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14(15)16/h2-13H2,1H3,(H,15,16)
Inchi Key
TUNFSRHWOTWDNC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
<li><strong>Safety assessment of myristic acid as a food ingredient.</strong> Myristic acid was evaluated for its safety as a food ingredient, demonstrating its compliance with health and safety standards and supporting its use in food manufacturing (Burdock and Carabin, 2007).</li>
<li><strong>Example of fatty acid-loaded lipoplex in enhancing in vitro gene transfer efficacies of cationic amphiphile.</strong> This research outlines how myristic acid can be used to improve the efficiency of gene delivery systems in medical research, suggesting a pivotal role in enhancing transfection efficiencies (Majeti et al., 2005).</li>
</ul>
Storage Class
11 - Combustible Solids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service