C4255
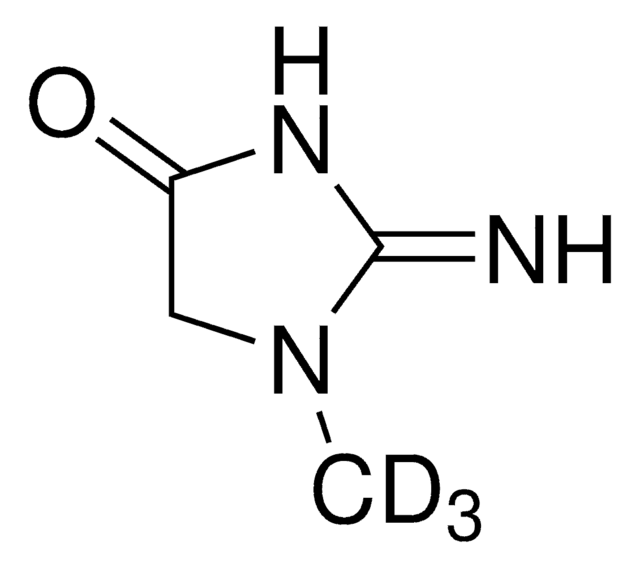
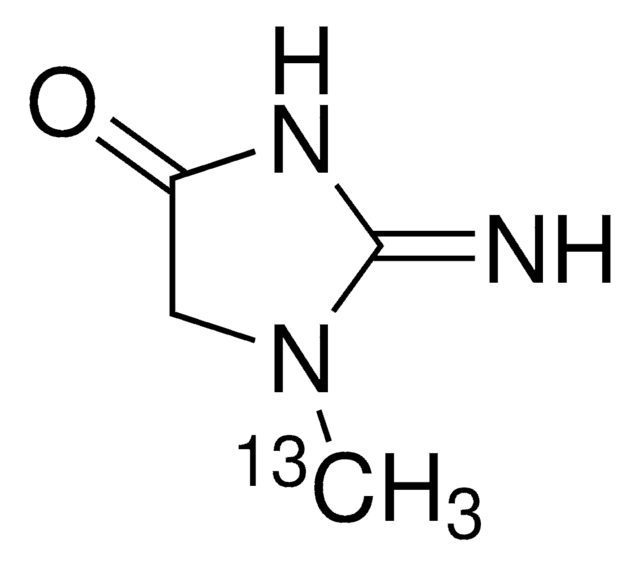
Creatinine
anhydrous, ≥98%
Synonym(s):
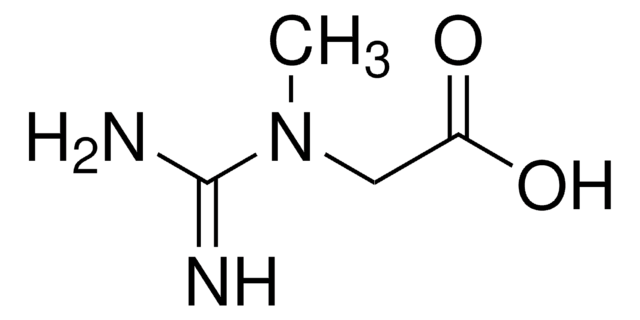
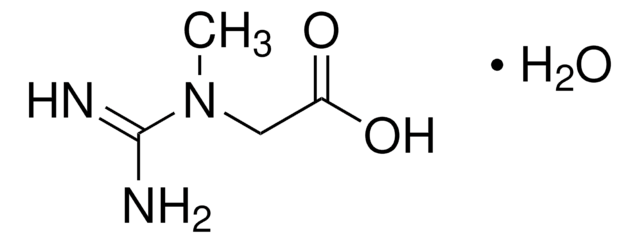
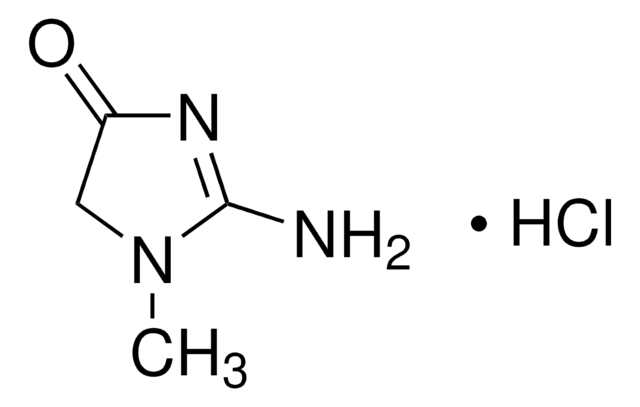
2-Amino-1-methyl-2-imidazolin-4-one, 2-Imino-1-methylimidazolidin-4-one, 2-Imino-N-methylhydantoin
About This Item
Recommended Products
grade
anhydrous
Quality Level
assay
≥98%
form
powder
mp
295 °C (dec.) (lit.)
SMILES string
CN1CC(=O)N=C1N
InChI
1S/C4H7N3O/c1-7-2-3(8)6-4(7)5/h2H2,1H3,(H2,5,6,8)
InChI key
DDRJAANPRJIHGJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- A nitrogen donor building block to prepare nitrogen-containing heterocyclic derivatives.[4][5]
- A reactant to synthesize donor-acceptor type carbon nitride copolymer, which is used as a photocatalyst in hydrogen production.[4]
- A starting material to prepare creatol(2-amino-1,5-dihydro-5-hydroxy methylimidazol-4-one) via creatinine chloramine.[6]
- A reactant to synthesize 3-substituted-3-hydroxyisatins by gold-catalyzed aldolization with various isatins.[7]
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
554.0 °F - closed cup
flash_point_c
290 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service