383120
Sulfamic acid
ACS reagent, 99.3%
Synonym(s):
Amidosulfonic acid
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
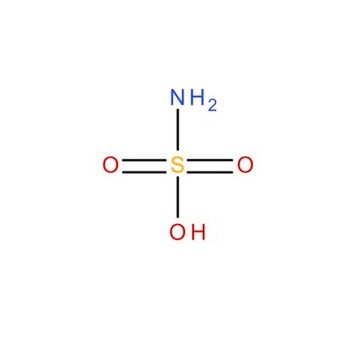
Linear Formula:
NH2SO3H
CAS Number:
Molecular Weight:
97.09
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
grade
ACS reagent
Quality Level
assay
99.3%
99.3-100.3% dry basis (ACS specification)
form
crystals
technique(s)
titration: suitable
impurities
≤0.01% insolubles
ign. residue
≤0.01%
mp
215-225 °C (dec.) (lit.)
solubility
water: 213 g/L at 20 °C
density
2.151 g/cm3 at 25 °C
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Sulfamic acid (H2NSO3H) is widely used inorganic compound. It exists in zwitterionic form and neutral forms. Zwitterionic form has been reported to be is more stable than neutral form. Its industrial applications have been reported. It has various organic synthesis applications.
Sulfamic acid is a strong inorganic acid. It is a hypochlorite scavenger. The ability of sulfamic acid to reduce nitrate to nitrogen gas under acidic condition has been utilized to denitrify nitrate-rich wastewater along with zinc powder.
Application
Sulfamic acid (H2NSO3H) may be used in the following studies:
- As catalyst in the synthesis of aryl-14H-dibenzo[a.j]xanthenes.
- As green catalyst for the preparation of amide from ketoxime.
- As ammonia equivalent in the regioselective synthesis of primary allylic amines, via allylic substitution reactions.
- Synthesis of polysubstituted quinolones.
Sulfamic acid may be used in the following processes:
- As a titrant in the determination of the burette injection volume and chemical calibration factor.
- To neutralize excess nitrous acid in the colorimetric paracetamol assay by modified Glynn and Kendal colorimetric method.
- To prevent endogenous mercury (Hg) loss during the urine Hg measurement by inductively coupled plasma mass spectrometry (ICP-MS) method.
- As an acid catalyst and a hypochlorite scavenger in the chlorite oxidation of dialdehyde cellulose (DAC).
- As a heterogeneous catalyst in the synthesis of polyhydroquinoline derivatives by Hantzsch condensation reaction.
- As catalyst in the degradation of bamboo fiber to 5-hydroxymethylfurfural (HMF).
- As an acid reagent in the determination of silicates in water samples based on centrifugal microfluidics.
- As catalyst in the synthesis of deazaoxaflavin at room temperature.
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class
8B - Non-combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Neil A Demarse et al.
Analytical biochemistry, 417(2), 247-255 (2011-07-12)
Obtaining accurate results with nanowatt titration calorimeters with overflow cells requires mass calibration of the buret injection volume, chemical calibration of the reaction vessel effective volume, and chemical calibration of the calorimetric factor used to convert the measured electrical signal
Hyundoo Hwang et al.
Analytical chemistry, 85(5), 2954-2960 (2013-01-17)
In this study, we describe a novel platform based on centrifugal microfluidics for simultaneous determination of nitrite, nitrate and nitrite, ammonium, orthophosphate, and silicate in water samples. All processes from sample metering to detection were integrated and automatically conducted on
Sulphamic acid assisted synthesis of polyhydroquinolines via Hantzsch multicomponent reaction: A green approach.
Lambat T, et al.
Journal of Chemical and Pharmaceutical Research, 6(4), 888-892 (2014)
Fathima Shihana et al.
Clinical toxicology (Philadelphia, Pa.), 48(1), 42-46 (2010-01-26)
Despite a significant increase in the number of patients with paracetamol poisoning in the developing world, plasma paracetamol assays are not widely available. The purpose of this study was to assess a low-cost modified colorimetric paracetamol assay that has the
Sulfamic Acid: Efficient, Cost-Effective Catalyst for Facile Synthesis of Deazaoxaflavin at Ambient Temperature.
Mane MM and Pore DM.
Synthetic Communications, 45(7), 868-876 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service