H6515
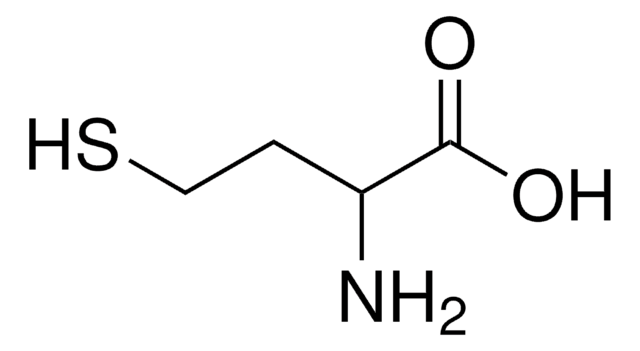
L-Homoserine
Synonym(s):
(S)-2-Amino-4-hydroxybutyric acid, Hse
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HOCH2CH2CH(NH2)CO2H
CAS Number:
Molecular Weight:
119.12
Beilstein/REAXYS Number:
1721681
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
assay
≥98% (TLC)
Quality Level
form
powder
color
white to off-white
mp
203 °C (dec.) (lit.)
application(s)
cell analysis
detection
SMILES string
N[C@@H](CCO)C(O)=O
InChI
1S/C4H9NO3/c5-3(1-2-6)4(7)8/h3,6H,1-2,5H2,(H,7,8)/t3-/m0/s1
InChI key
UKAUYVFTDYCKQA-VKHMYHEASA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
L-Homoserine is a variant of serine with an additional carbon on its side chain.
Application
L-Homoserine has been used as an internal standard for neurotransmitter analysis and amino acids quantification.
Biochem/physiol Actions
L-Homoserine is synthesized by deoxidation process, catalysed by homoserine dehydrogenase. This is one of the steps in the synthesis of L-threonine. The carbon flux in in bacteria such as E. coli is maintained by this reaction.
L-Homoserine is used in the biosynthesis of methionine, threonine and isoleucine.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Minsang Lee et al.
The Plant journal : for cell and molecular biology, 41(5), 685-696 (2005-02-11)
Homoserine kinase (HSK) produces O-phospho-l-homoserine (HserP) used by cystathionine gamma-synthase (CGS) for Met synthesis and threonine synthase (TS) for Thr synthesis. The effects of overexpressing Arabidopsis thaliana HSK, CGS, and Escherichia coli TS (eTS), each controlled by the 35S promoter
Quantification of 3-deoxyglucosone (3DG) as an aging marker in natural and forced aged wines
Oliveira CM, et al.
J. Food Compos. Anal., 70?76-70?76 (2016)
D C Turnell et al.
Clinical chemistry, 28(3), 527-531 (1982-03-01)
This method for estimating clinically important amino acids in serum or urine within 40 min involves o-phthalaldehyde/2-mercaptoethanol derivatization and reversed-phase "high-pressure" liquid chromatography. Homocysteic acid is an internal standard, and homoserine and norvaline are reference peaks. For all the amino
Varnika Roy et al.
Applied microbiology and biotechnology, 97(6), 2627-2638 (2012-10-12)
Quorum sensing (QS), the process of autoinducer-mediated cell-cell signaling among bacteria, facilitates biofilm formation, virulence, and many other multicellular phenotypes. QS inhibitors are being investigated as antimicrobials because of their potential to reduce symptoms of infectious disease while slowing the
Minhao Wu et al.
The Journal of biological chemistry, 288(22), 15878-15887 (2013-04-17)
Quorum-sensing systems are widely used by bacteria to control behavior in response to fluctuations in cell density. Several small diffusible molecules called autoinducers act as signaling molecules in quorum-sensing processes through interplay with sensors. Autoinducers modulate vital physiological functions such
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service