I2764
ML-7
powder
Synonym(s):
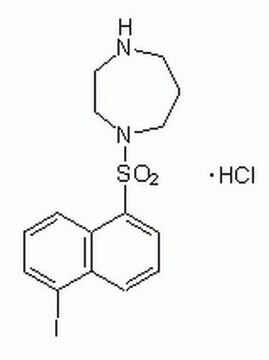
1-(5-Iodonaphthalene-1-sulfonyl)-1H-hexahydro-1,4-diazepine hydrochloride
About This Item
Recommended Products
assay
≥98% (TLC)
Quality Level
form
powder
color
white
solubility
ethanol: water (1:1): 10 mg/mL, clear, colorless to faintly yellow
storage temp.
−20°C
SMILES string
Cl.Ic1cccc2c(cccc12)S(=O)(=O)N3CCCNCC3
InChI
1S/C15H17IN2O2S.ClH/c16-14-6-1-5-13-12(14)4-2-7-15(13)21(19,20)18-10-3-8-17-9-11-18;/h1-2,4-7,17H,3,8-11H2;1H
InChI key
KDDALCDYHZIZMH-UHFFFAOYSA-N
Gene Information
human ... MYLK(4638) , MYLK2(85366)
Application
Biochem/physiol Actions
Features and Benefits
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service