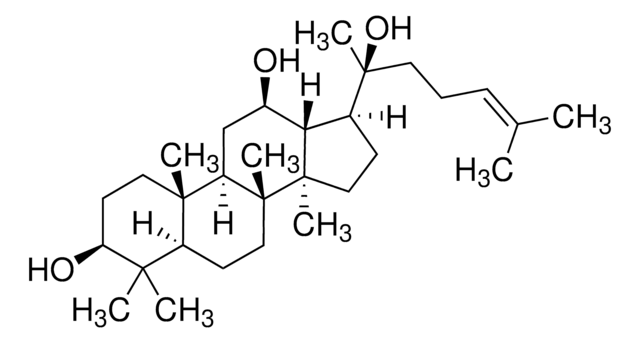
SMB00348
Ginsenoside Compound K
≥96% (HPLC)
Synonym(s):
(3B,12B)-3,12-Dihydroxydammar-24-en-20-yl β-D-glucopyranoside, 20(S)-Ginsenoside Ck, Ginsenoside K, Protopanaxadiol 20-O-glucoside
About This Item
Recommended Products
assay
≥96% (HPLC)
form
powder
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
SMILES string
CC(C)=CCC[C@](C1C2[C@H](O)CC3[C@@]([C@]2(C)CC1)(C)CCC4C(C)(C)[C@@H](O)CC[C@@]43C)(C)O[C@@H]5O[C@H](CO)[C@@H](O)[C@H](O)[C@H]5O
InChI
1S/C36H62O8/c1-20(2)10-9-14-36(8,44-31-30(42)29(41)28(40)23(19-37)43-31)21-11-16-35(7)27(21)22(38)18-25-33(5)15-13-26(39)32(3,4)24(33)12-17-34(25,35)6/h10,21-31,37-42H,9,11-19H2,1-8H3/t21?,22-,23-,24?,25?,26+,27?,28-,29+,30-,31+,33+,34-,35-,36+/m1/s1
InChI key
FVIZARNDLVOMSU-KJULZEBLSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Combination of Electrochemistry and Mass Spectrometry to Study Nitric Oxide Metabolism and Its Modulation by Compound K in Breast Cancer Cells.: This research explores the interaction of Ginsenoside Compound K with nitric oxide metabolism in breast cancer cells, employing a combination of electrochemical techniques and mass spectrometry to elucidate the metabolic pathways and potential therapeutic effects of Compound K (Zhao et al., 2022).
- Metabolic analysis of Panax notoginseng saponins with gut microbiota-mediated biotransformation by HPLC-DAD-Q-TOF-MS/MS.: This article investigates the gut microbiota-mediated biotransformation of Panax notoginseng saponins, including Compound K, detailing the complex interactions and metabolic profiles in a biological system, which could inform their therapeutic use and bioavailability (Chen et al., 2018).
Biochem/physiol Actions
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service