SML1779
Nigericin sodium salt
from Streptomyces hygroscopicus, ≥98% (HPLC), solution, polyether ionophore
Synonym(s):
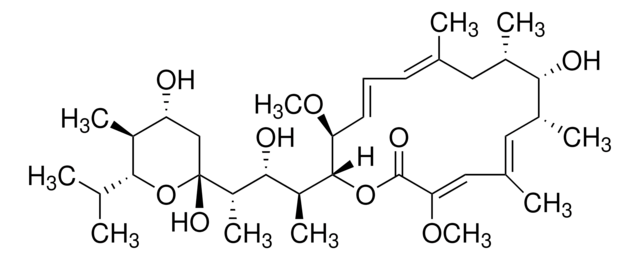
3B2-6379, Antibiotic K178, Antibiotic X464, Azalomycin M, HE331800, Helexin C, Polyetherin A, sodium;2-[(3S,6R)-6-[[(5R,6R,7R,9R)-2-[5-[(3S,5R)-5-[(2S,3S,5R,6R)-6-hydroxy-6-(hydroxymethyl)-3,5-dimethyloxan-2-yl]-3-methyloxolan-2-yl]-5-methyloxolan-2-yl]-7-methoxy-2,4,6-trimethyl-1,10-dioxaspiro[4.5]decan-9-yl]methyl]-3-methyloxan-2-yl]propanoate
Select a Size
Select a Size
About This Item
Recommended Products
Product Name
Nigericin sodium salt Ready Made Solution, 5 mg/mL (DMSO:ethanol 1:1)
biological source
Streptomyces hygroscopicus
Quality Level
form
solution
concentration
5 mg/mL (DMSO:ethanol 1:1)
antibiotic activity spectrum
Gram-positive bacteria
mode of action
cell membrane | interferes
shipped in
ambient
storage temp.
2-8°C
SMILES string
[Na+].[O-]C(=O)C(C1O[C@H](CC[C@@H]1C)C[C@H]2O[C@]3(OC(CC3C)(C4OC(CC4)(C5O[C@H](C[C@@H]5C)[C@H]6O[C@@]([C@@H](C[C@@H]6C)C)(O)CO)C)C)[C@@H]([C@@H](C2)OC)C)C
Related Categories
1 of 4
This Item | CLS3591 | CLS3797 | CLS3590 |
|---|---|---|---|
| suitability suitable for (EIA/RIA; immunoassays) | suitability suitable for (EIA/RIA; immunoassays) | suitability suitable for (EIA/RIA; immunoassays) | suitability suitable for (EIA/RIA; immunoassays) |
| material clear bottom, flat bottom wells, round wells, clear wells, polystyrene | material flat bottom clear , polystyrene , round wells | material clear bottom, clear polystyrene , round bottom , round wells | material flat bottom clear , polystyrene , round wells |
| sterility non-sterile | sterility non-sterile | sterility non-sterile | sterility non-sterile |
| feature lid: no | feature lid: no, skirt, plate format: 96 well standard, well: flat bottom, clear | feature lid: no, skirt, plate format: 96 well standard | feature lid: no, skirt, plate format: 96 well standard, well: flat bottom, clear |
| packaging case of 100 | packaging pack of 1, case of 50, pkg of (individually wrapped) | packaging case of 100, bag of 25 | packaging pack of 1, case of 100, pkg of (individually wrapped) |
Biochem/physiol Actions
Nigericin kills bacteria by facilitating the diffusion of ions across membranes.
Low concentration (0.5 μM) of Nigericin rapidly decreases pHi, causing stimulation of PG production 1.5- to 2-fold in cerebral microvascular endothelial cells and arresting of DNA synthesis in Erlich acites carcinoma cells. Treatment of Hela cells, after entry of poliovirus, with nigericin, prevents the inhibition of host protein synthesis by poliovirus. Nigericin is also widely used in studies of the consequences of changes in membrane potential in variable systems.
Preparation Note
Storage and Stability
signalword
Warning
hcodes
Hazard Classifications
Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
78.8 °F
flash_point_c
26 °C
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service