T2807
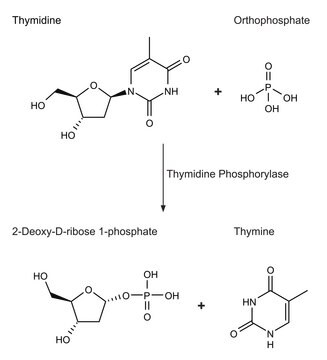
Thymidine Phosphorylase, recombinant from Escherichia coli
recombinant, expressed in E. coli, buffered aqueous solution, ≥500 units/mL
Synonym(s):
Thymidine:orthophosphate deoxy-D-ribosyltransferase
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
form
buffered aqueous solution
concentration
≥500 units/mL
UniProt accession no.
storage temp.
2-8°C
Gene Information
Escherichia coli K12 ... deoA(948901)
Looking for similar products? Visit Product Comparison Guide
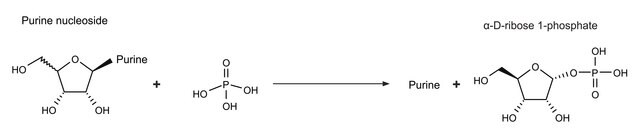
General description
Application
Biochem/physiol Actions
Unit Definition
Physical form
Preparation Note
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service