T6137
Trioxsalen
≥98% (HPLC), powder, photochemical DNA crosslinker
Synonym(s):
2,5,9-Trimethylfuro[3,2-g]benzopyran-7-one, 4,5′,8-Trimethylpsoralen, TMP, Trisoralen
About This Item
Recommended Products
Product Name
Trioxsalen, ≥98% (HPLC), powder
Quality Level
assay
≥98% (HPLC)
form
powder
color
white
mp
229-231 °C (lit.)
solubility
chloroform: soluble 50 mg/mL, clear, colorless to faintly yellow
fluorescence
λex 269 nm; λem 445 nm in methanol
λex 321 nm; λem 445 nm (bound to DNA in Tris, pH 8.1)
originator
Valeant
storage temp.
−20°C
SMILES string
Cc1cc2cc3C(C)=CC(=O)Oc3c(C)c2o1
InChI
1S/C14H12O3/c1-7-4-12(15)17-14-9(3)13-10(6-11(7)14)5-8(2)16-13/h4-6H,1-3H3
InChI key
FMHHVULEAZTJMA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- to induce small deletion mutations in worms
- in combination with ultraviolet A (UVA)
- to induce interstrand crosslinks (ICLs) in DNA
- for the preparation and photoactivation of trimethyl psoralen
Biochem/physiol Actions
Features and Benefits
Preparation Note
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Eye Dam. 1 - Muta. 2 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Apoptosis regulation involves multiple pathways and molecules for cellular homeostasis.
Cell cycle phases (G1, S, G2, M) regulate cell growth, DNA replication, and division in proliferating cells.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service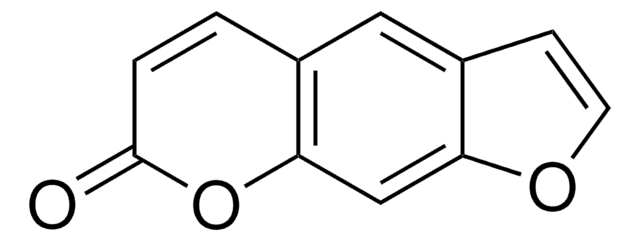










![2-Amino-9H-pyrido[2-3-b]indole ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/351/152/37c98523-590f-4b27-826c-5b3d4b502047/640/37c98523-590f-4b27-826c-5b3d4b502047.png)

