LC/MS/MS Analysis of Interacting Cardiac Drugs Digoxin, Quinidine, Amiodarone and Verapamil on Titan C18
Materials
SPE tube or plate
CONDITIONS
sample/matrix
Sample spiked into rat plasma
SPE well plate
HybridSPE®-PLus 96-Well Plate (575659-U)
condition
100 uL of spiked rat plasma was added to the HybridSPE plate well, followed by 300 uL of protein precipitation solvent, acetonitrile with 1% formic acid. (The plate, sealed, secured on a vibrater, agitated by vibration at 1000 rpm for 2 min. The plate was transfered to a vacuum manifold. Vacuum was applied at 10 in Hg for 4 min.)
column
Titan C18, 5 cm x 2.1 mm I.D., 1.9 μm particles (577122-U)
mobile phase
[A] Water; [B] methanol, both with 0.1% formic acid/10 mM ammonium formate
gradient
0-1 min, 45%B; 1.5-4 min 90%B, 4.5-8 min 45%B
flow rate
0.2 mL/min
pressure
2900 psi
column temp.
35 °C
detector
MS, ESI(+), MRM mode
injection
1 μL
Description
Analysis Note
Sample pretreatment: Sample spiked into rat plasma
Detector:
MRM transitions:
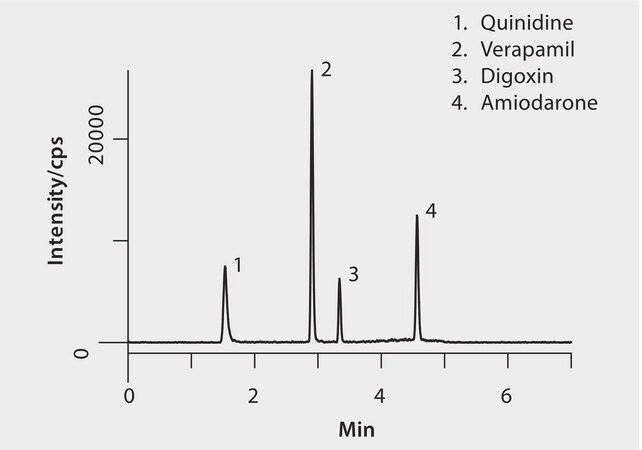
Peak MRM
1. Quinidine325.2/81.1, 307.1
2. Verapamil455.3/165.1
3. Digoxin798.5/651.5
4. Amiodarone646.1/58.1, 100.1
Legal Information
HybridSPE is a registered trademark of Merck KGaA, Darmstadt, Germany
null