PEPPSI™-IPent for Demanding Cross-Coupling Reactions
Introduction
Professor Mike Organ and co-workers have developed the PEPPSI™ (Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation) precatalysts for palladium-catalyzed cross-coupling reactions. PEPPSI™ palladium precatalysts have become a choice alternative to palladium phosphine complexes, as they are comprised of a bulky N-heterocyclic-carbene (NHC) ligand bonded to palladium and a s-donating 3-chloropyridine ligand for stability. The PEPPSI™-IPent (732117) further advances the agility of cross-coupling reactions by being air-stable and thus increasing user-friendliness. We are proud to offer the most active of the PEPPSI™ series, PEPPSI™-IPent, in collaboration with the Organ Research Group.1
Advantages
- PEPPSI™-IPent is bench stable: no need for a glovebox
- Poisoning of Pd has not been observed
- Higher pyrrole-based cross-coupling reactions success
- Consistently superior coupling yields over PEPPSI™-IPr
- Effective and versatile catalyst for difficult, sterically-demanding reactions2
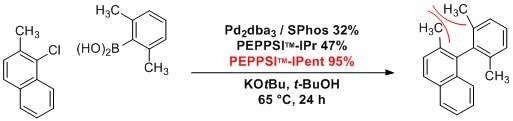
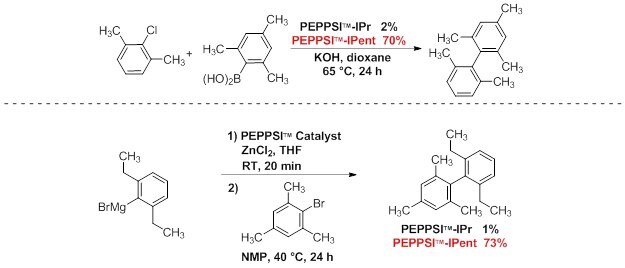
Representative Applications
Under relatively mild conditions, PEPPSI™-IPent is a highly effective catalyst in challenging Suzuki–Miyaura and Negishi cross-coupling reactions. This produces tetra-ortho-substituted (hetero)biaryl compounds in high yields, especially when compared to the use of PEPPSI™-IPr. Bulky and acidic substituents were observed to be well tolerated.1,2
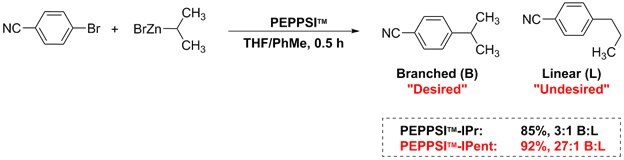
Negishi cross-coupling reactions of secondary alkyl zinc halides with aryl halides utilize PEPPSI™-IPent, boasting higher selectivity than PEPPSI™-IPr. The desired branched product was obtained in higher yields than that of the linear product.1,3
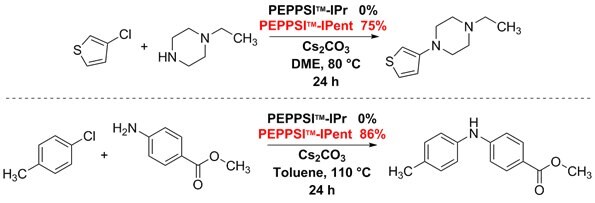
Buchwald–Hartwig–Yagupol’skii aryl aminations typically use strong bases to aid the rate-limiting step, creating conditions that functional groups poorly tolerate. In contrast, using mildly basic conditions and PEPPSI™-IPent, a variety of demanding aryl chlorides were successfully coupled to secondary amines. In addition, PEPPSI™-IPent facilitated the coupling of electron-deficient anilines with electron-rich aryl chlorides. In these reactions, the highly reactive PEPPSI™-IPent consistently outperformed PEPPSI™-IPr.1,4,5
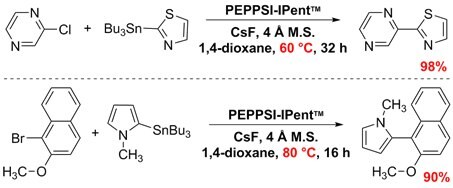
In Stille–Migita cross-couplings of heteroaryl stannanes with aryl halides, PEPPSI™-IPent allowed for lower reaction temperatures below 100 ºC for thermally sensitive cross-coupling partners. Organ and co-workers observed successful coupling within in the range of 30–80 ºC while maintaining consistent yields.1,6
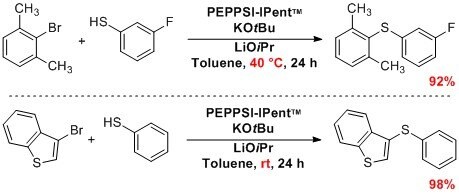
High temperatures frequently used for carbon-sulfur bond formation were circumvented with PEPPSI™-IPent, featuring mild conditions for the formation of thiol ethers by metal-catalyzed sulfinations. Successful reactions have included the sulfination of (hetero)aryl halides with aryl sulfides, alkyl thiols, and also sterically hindered substrates.1,7
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?