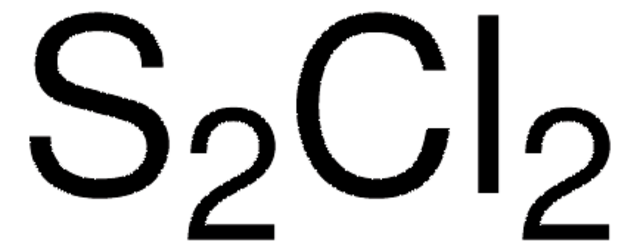すべての画像(1)
About This Item
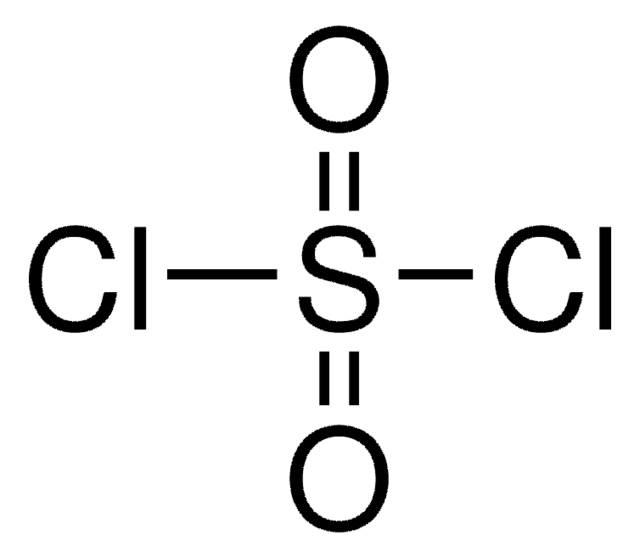
化学式:
SO2Cl2
CAS番号:
分子量:
134.97
MDL番号:
UNSPSCコード:
12352300
PubChem Substance ID:
NACRES:
NA.23
フォーム:
liquid
おすすめの製品
フォーム
liquid
品質水準
濃度
1.0 M in methylene chloride
密度
1.352 g/mL at 25 °C
SMILES記法
ClS(Cl)(=O)=O
InChI
1S/Cl2O2S/c1-5(2,3)4
InChI Key
YBBRCQOCSYXUOC-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
アプリケーション
- Palladium-Catalyzed Synthesis of Ammonium Sulfinates from Aryl Halides and a Sulfur Dioxide Surrogate: A Gas-and Reductant-Free Process: This study elaborates on using sulfuryl chloride for oxidative chlorination leading to sulfonyl chloride formation, which is important for synthetic organic chemistry (Emmett et al., 2014).
- Combining Organometallic Reagents, the Sulfur Dioxide Surrogate DABSO, and Amines: A One‐Pot Preparation of Sulfonamides, Amenable to Array Synthesis: This research discusses an innovative one-pot method for preparing sulfonamides using sulfuryl chloride, highlighting its utility in streamlining complex syntheses (Deeming et al., 2015).
- Interfacial polymerization: from chemistry to functional materials: The article illustrates the use of sulfuryl chloride in interfacial polymerization, a key technique for developing advanced materials with tailored properties (Zhang et al., 2020).
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Inhalation - Carc. 2 - Eye Dam. 1 - Skin Corr. 1C - STOT SE 3
ターゲットの組織
Central nervous system
補足的ハザード
保管分類コード
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
PRTR
第一種指定化学物質
労働安全衛生法名称等を表示すべき危険物及び有害物
名称等を表示すべき危険物及び有害物
労働安全衛生法名称等を通知すべき危険物及び有害物
名称等を通知すべき危険物及び有害物
Jan Code
278505-VAR:
278505-BULK:
278505-800ML:4548173225388
278505-100ML:4548173225371
この製品を見ている人はこちらもチェック
Manli Zhang et al.
Chemical communications (Cambridge, England), 47(41), 11522-11524 (2011-09-29)
A palladium-catalyzed direct desulfitative C-arylation of a benzo[d]oxazole C-H bond with arene sulfonyl chlorides is described. The procedure tolerates halo, cyano, nitro, trifluoromethyl, acetyl and acetylamino groups on the phenyl ring of sulfonyl chlorides, providing the arylation products in moderate
Jianbin Chen et al.
Chemical communications (Cambridge, England), 48(3), 449-451 (2011-11-15)
A palladium-catalyzed desulfitative cyanation of arenesulfonyl chlorides and sodium sulfinates has been developed, providing aryl nitriles in moderate to excellent yields. It represents a facile procedure to access aryl nitriles.
Qian Wu et al.
Chemical communications (Cambridge, England), 47(32), 9188-9190 (2011-07-14)
A new, efficient protocol for the synthesis of di(hetero)aryl sulfides is described. Cheap and easily available arylsulfonyl chlorides as a sulfur source reductively couple with electron-rich (hetero)arenes (e.g., indolizines, indoles, electron-rich benzenes, etc.) in the presence of triphenylphosphine to afford
Philippe Hermange et al.
Organic letters, 11(18), 4044-4047 (2009-08-15)
Reaction of an isoquinoline, a silyloxyfuran, and an acyl or sulfonyl chloride provides easy access to a wide variety of isoquinolinobutyrolactones with excellent yields and diastereoselectivites (R*,R* isomer), even in the case of formation of quaternary centers (i.e., R(3) or
Christopher Blackburn
ACS combinatorial science, 14(3), 150-154 (2012-02-11)
Reductive aminations and further transformations of an azo dye and fluorous tagged aldehyde are described. The intensely colored 2,4-dialkoxybenzyl protected amines undergo Fmoc-based peptide coupling, Suzuki reactions, and sulfonamide formation with product isolation facilitated by visual monitoring of fluorous solid
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)