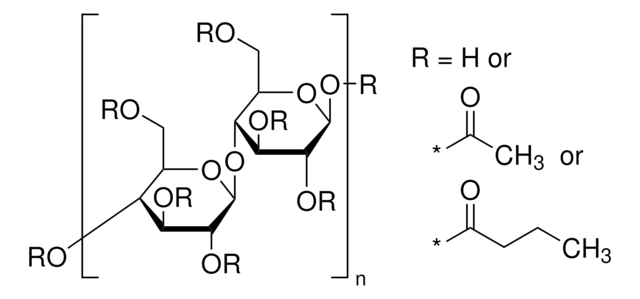
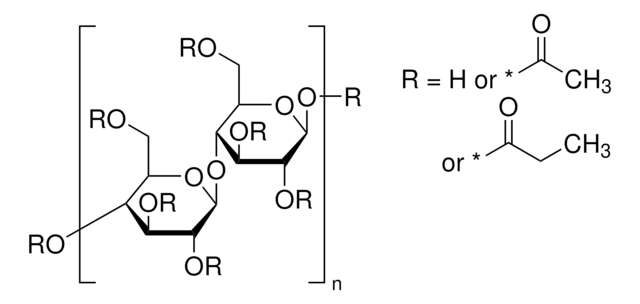
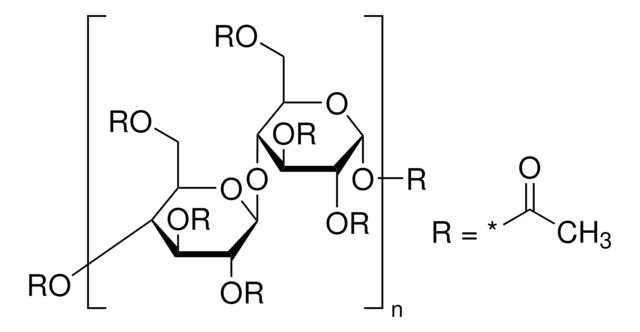
おすすめの製品
形状
powder
品質水準
分子量
average Mn ~70,000
標識化の程度
12-15 wt. % Acetyl
35-39 wt. % Butyryl
1.2-2.2 wt. % Hydroxyl
屈折率
n20/D 1.475 (lit.)
密度
1.25 g/mL at 25 °C (lit.)
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
保管分類コード
11 - Combustible Solids
WGK
nwg
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
419044-250G:
419044-BULK:
419044-VAR:
419044-500G:
この製品を見ている人はこちらもチェック
Gheorghe Fundueanu et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 66(1), 11-20 (2006-11-07)
The aim of this work is to safely transport bioadhesive microspheres loaded with DNA to intestine and to test their bioadhesive properties. Poly(vinyl alcohol) (PVA) microspheres were prepared by dispersion reticulation with glutaraldehyde and further aminated. These microspheres were firstly
Chenyang Xing et al.
Carbohydrate polymers, 92(2), 1921-1927 (2013-02-13)
The eco-friendly poly(propylene carbonate) (PPC)/cellulose acetate butyrate (CAB) blends were prepared by melt-blending in a batch mixer for the first time. PPC and CAB were partially miscible because of the drastically shifted glass transition temperatures of both PPC and CAB
Mitra Jelvehgari et al.
Archives of pharmacal research, 32(7), 1019-1028 (2009-07-31)
The present research work compares the effect of microsphere preparation technique on micromeritics and release behaviors of theophylline microspheres. Microspheres were prepared by oil-in oil (O(1)/O(2)) emulsion solvent evaporation method (ESE) using different ratios of anhydrous theophylline to cellulose acetate
M Constantin et al.
International journal of pharmaceutics, 330(1-2), 129-137 (2006-10-10)
Poly(vinyl alcohol) (PVA) microspheres were prepared by dispersion reticulation with glutaraldehyde and further aminated. These microspheres were firstly loaded with diclofenac (DF) and then entrapped in cellulose acetate butyrate (CAB) microcapsules by an o/w solvent evaporation technique for intestinal delivery
Wasfy M Obeidat et al.
Journal of microencapsulation, 24(3), 263-273 (2007-04-25)
The dispersion/incorporation of Eudragit S100 powder as a filler in cellulose acetate butyrate (CAB-551-0.01) microsphere containing theophylline was investigated as a means of controlling drug release. Microspheres of CAB-551-0.01 of different polymer solution concentrations/viscosities were prepared (preparations Z(0), Z(A), Z(B)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)