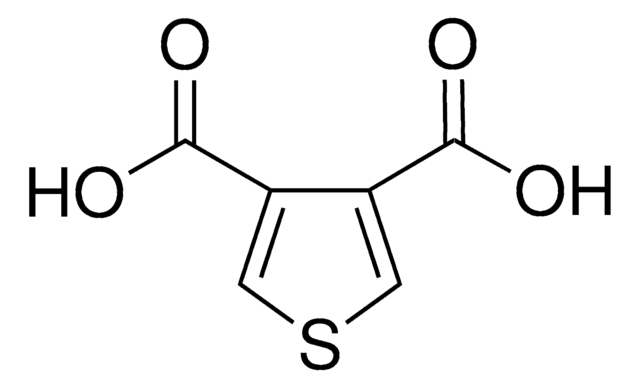
おすすめの製品
アッセイ
≥97%
品質水準
フォーム
powder
SMILES記法
[s]1c(ccc1c2[s]c(c4c2C(=O)c5c(c([s]c5CC(CCCC)CC)CC(CCCC)CC)C4=O)c3[s]c(cc3)Br)Br
InChI
1S/C34H38Br2O2S4/c1-5-9-11-19(7-3)17-23-27-28(24(39-23)18-20(8-4)12-10-6-2)32(38)30-29(31(27)37)33(21-13-15-25(35)40-21)42-34(30)22-14-16-26(36)41-22/h13-16,19-20H,5-12,17-18H2,1-4H3
関連するカテゴリー
アプリケーション
1,3-Bis(5-bromo-2-thienyl)-5,7-bis(2-ethylhexyl)-4H,8H-benzo[1,2-c:4,5-c′]dithiophene-4,8-dione (BDD), a strong electron accepting molecule, can be used in the fabrication of non-fullerene polymeric solar cells. It can also be used in the synthesis of a new conjugated polymer, 5,7-bis(2- ethylhexyl)benzo[1,2-c:4,5-c′]dithiophene-4,8-dione (PBDTBDD).
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
High-efficiency non-fullerene polymer solar cell fabricated by a simple process using new conjugated terpolymers
Hoang MH, et al.
Journal of Material Chemistry C, 7(1), 111-118 (2019)
Wenchao Zhao et al.
Journal of the American Chemical Society, 139(21), 7148-7151 (2017-05-18)
A new polymer donor (PBDB-T-SF) and a new small molecule acceptor (IT-4F) for fullerene-free organic solar cells (OSCs) were designed and synthesized. The influences of fluorination on the absorption spectra, molecular energy levels, and charge mobilities of the donor and
Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells.
Zhao W, et al.
Journal of the American Chemical Society, 139(21), 7148-7151 (2017)
Single-junction organic solar cells based on a novel wide-bandgap polymer with efficiency of 9.7%.
Lijun Huo et al.
Advanced materials (Deerfield Beach, Fla.), 27(18), 2938-2944 (2015-04-03)
Design, Application, and Morphology Study of a New Photovoltaic Polymer with Strong Aggregation in Solution State.
Qian D, et al.
Macromolecules, 45(24), 9611-9617 (2012)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)![1,1′-[4,8-Bis[5-(2-ethylhexyl)-2-thienyl]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]](/deepweb/assets/sigmaaldrich/product/structures/611/912/a638a6fe-ca7b-4674-8023-df4c0921a9fd/640/a638a6fe-ca7b-4674-8023-df4c0921a9fd.png)
![4,7-ジブロモベンゾ[c]-1,2,5-チアジアゾール 95%](/deepweb/assets/sigmaaldrich/product/structures/711/964/3fd3ffd1-5916-468e-a743-22f1611b5a33/640/3fd3ffd1-5916-468e-a743-22f1611b5a33.png)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene ≥97%](/deepweb/assets/sigmaaldrich/product/structures/287/437/cf540b93-ec8c-4d2a-897c-dea0a28a8def/640/cf540b93-ec8c-4d2a-897c-dea0a28a8def.png)
![2,5-ジヒドロ-3,6-ジ-2-チエニル-ピロロ[3,4-c]ピロール-1,4-ジオン 97%](/deepweb/assets/sigmaaldrich/product/structures/209/681/63a4048f-a2a7-496b-814d-ccb4b5b76124/640/63a4048f-a2a7-496b-814d-ccb4b5b76124.png)
![2,5-Bis(trimethylstannyl)-thieno[3,2-b]thiophene 97%](/deepweb/assets/sigmaaldrich/product/structures/126/532/26557e94-858e-4c96-90de-ca88d84a8727/640/26557e94-858e-4c96-90de-ca88d84a8727.png)
![ベンゾ[1,2-b:4,5-b′]ジチオフェン-4,8-ジオン 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)
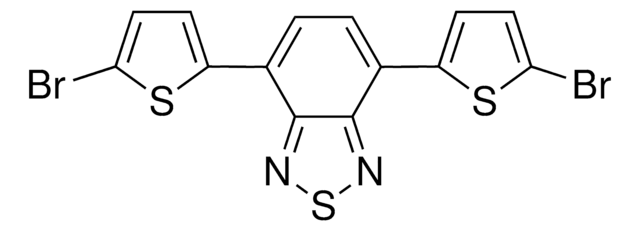
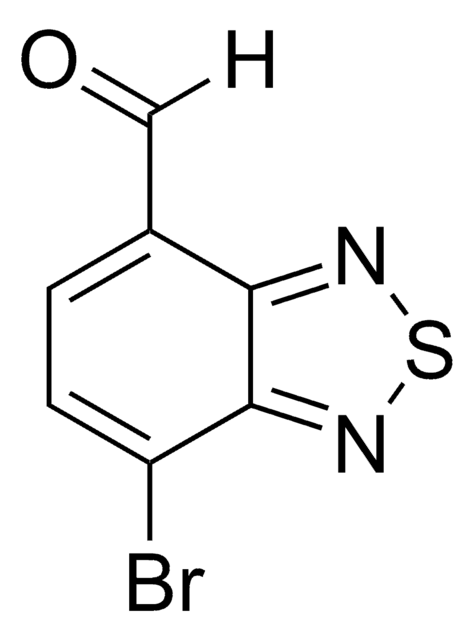
![3,6-ビス(5-ブロモ-2-チエニル)-2,5-ビス(2-ヘキシルデシル)-2,5-ジヒドロ-ピロロ[3,4-c]ピロール-1,4-ジオン 98%](/deepweb/assets/sigmaaldrich/product/structures/128/499/590a62c1-529b-42e2-96df-25659ec8c9e0/640/590a62c1-529b-42e2-96df-25659ec8c9e0.png)