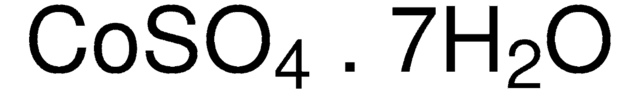おすすめの製品
タイプ
(High purity Salts)
品質水準
アッセイ
≥99.99% trace metals basis
98-102% (EDTA, complexometric)
フォーム
powder or crystals
不純物
≤1000 ppm trace metals basis
色
faint blue to dark blue-green
pH
4.3-4.7 (20 °C, 100 g/L in water)
溶解性
water: soluble
密度
2.07 g/cm3 at 20 °C
微量陰イオン
chloride (Cl-): ≤20 ppm
微量陽イオン
Al: <50 ppm
Ca: <50 ppm
Co: <50 ppm
Cu: <50 ppm
Fe: <50 ppm
K: <50 ppm
Mg: <50 ppm
Na: <50 ppm
Pb: <50 ppm
Zn: <50 ppm
SMILES記法
[Ni+2].[S](=O)(=O)([O-])[O-].O.O.O.O.O.O
InChI
1S/Ni.H2O4S.6H2O/c;1-5(2,3)4;;;;;;/h;(H2,1,2,3,4);6*1H2/q+2;;;;;;;/p-2
InChI Key
RRIWRJBSCGCBID-UHFFFAOYSA-L
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
アプリケーション
Therefore, Nickel(II) sulfate hexahydrate has been widely used as a key component for the synthesis of Cathode for Lithium-ion batteries. - Spherical NCM 622 and Li[Ni0.9Co0.05Mn0.05]O2 (NCM 900505) were synthesized via a co-precipitation method using Nickel(II) sulfate hexahydrate. In order to achieve the desired energy density, it is necessary to maximize both the nickel content in the cathode and the cutoff voltage. [] - to synthesis single-crystal, Ni-rich NCM and polycrystalline NCM cathodes with various Ni content by coprecipitation method . These Ni-rich layered cathodes like NCM, NCA, and NCMA ([Ni1–x–yCox(Mn and/or Al)y]O2) are the top choices for powering upcoming electric vehicles. It is found that polycrystalline NCM cathodes are prone to intergranular microcracking during cycling, single crystal NCM cathodes demonstrate resilience against mechanical fracture, even under highly charged conditions or repeated cycles. Due to limited lithium-ion diffusion pathways, the electrochemical performance of single crystal -NCM cathodes, particularly in terms of capacity and cycling stability, is lower compared to that of polycrystalline-NCM cathodes. The difference in the electrochemical performance of single crystal -NCM and polycrystalline-NCM cathodes grows as the Ni fraction increases. In addition, Nickel(II) sulfate hexahydrate is widely used for electroplating for producing metallic coatings. Nickel(II) sulfate hexahydrate can also be used as a catalyst in: -Pt50Ni50 catalysts supported on MCM-41 were produced using wet co-impregnation. These catalysts were then employed for hydrogenation reactions of benzene in gas phase. The morphology of the metal phase within the catalysts has a notable impact on the conversion of benzene to cyclohexane. Factors like reduction temperature, NaBH4 concentration, and reduction medium influence the particle morphology. []
特徴および利点
- Water soluble
- Medium purity (99.9%)
- Low trace metals in ppm level
- Cost effective Suitable for battery applications
- Recycled catalyst
シグナルワード
Danger
危険有害性の分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A Inhalation - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 1 Inhalation
ターゲットの組織
Respiratory Tract
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
939331-250G:
939331-VAR:
939331-100G:
939331-BULK:
最新バージョンのいずれかを選択してください:
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)