おすすめの製品
フォーム
powder
包装
pkg of 1 × 10 mg (860602P-10mg)
pkg of 1 × 5 mg (860602P-5mg)
メーカー/製品名
Avanti Research™ - A Croda Brand 860602P
脂質タイプ
sphingolipids
輸送温度
dry ice
保管温度
−20°C
SMILES記法
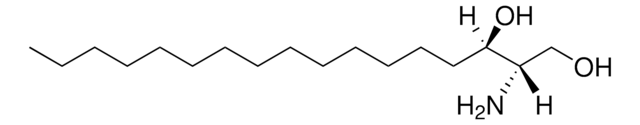
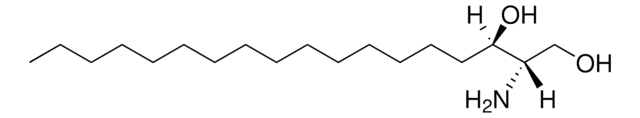
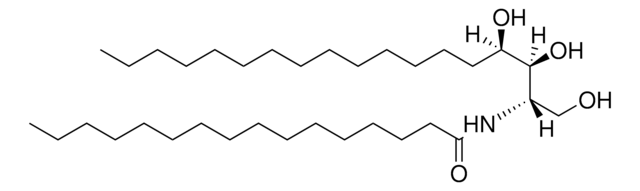
O[C@]([H])(CCCCCCCCCCCCC)[C@](O)([H])[C@](N)([H])CO
詳細
D-ribo-phytosphingosine (C17 base), also known as 4D-hydroxysphinganine or PHS, is widely found in membranes of fungi, plants, bacteria, marine organisms and mammalian tissues.
アプリケーション
D-ribo-phytosphingosine (C17 base) has been used as a standard for the quantification of total plant long-chain bases (LCB) by gas chromatography-mass spectrometry (GC-MS).
生物化学的/生理学的作用
D-ribo-phytosphingosine helps in maintaining the structural integrity of membrane. It also controls cellular growth and mediates the heat stress response of yeast. In addition, PHS acts as a precursor for synthesis of various key lipid mediators including PHS 1-phosphate, inositol phosphorylceramide and KRN7000 (α-anomer of galactosylceramide). This phospholipid also has an ability to stimulate keratinocyte differentiation. Therefore, PHS is used as an active constituent in cosmetic formulations.
包装
5 mL Amber Glass Screw Cap Vial (860602P-10mg)
5 mL Amber Glass Screw Cap Vial (860602P-5mg)
法的情報
Avanti Research is a trademark of Avanti Polar Lipids, LLC
保管分類コード
11 - Combustible Solids
WGK
WGK 3
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
860602P-5MG:
860602P-VAR:
860602P-10MG:
860602P-BULK:
最新バージョンのいずれかを選択してください:
Polar emollients in cosmetic formulations enhance the penetration and biological effects of Phytosphingosine on skin
Schiemann Y, et al.
Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 331(1-2), 103-107 (2008)
Asymmetric synthesis of d-ribo-phytosphingosine from 1-tetradecyne and (4-methoxyphenoxy) acetaldehyde
Liu Z, et al.
The Journal of Organic Chemistry, 75(13), 4356-4364 (2010)
Zheng Liu et al.
The Journal of organic chemistry, 75(13), 4356-4364 (2010-06-10)
An asymmetric synthesis of d-ribo-phytosphingosine (1) was achieved by utilizing the ProPhenol (12)-catalyzed alkynylation of unsaturated aldehyde 8 to afford allylic propargylic alcohol (S)-6 followed by asymmetric epoxidation and opening of propargylic epoxy alcohol anti-5 with NaN(3)/NH(4)Cl. Deprotection and reduction
Jean-Luc Cacas et al.
Analytical and bioanalytical chemistry, 403(9), 2745-2755 (2012-05-12)
In eukaryotic organisms, sphingolipids are major structural lipids of biological membranes and perform additional essential functions as signalling molecules. While long-chain bases (LCB), the common precursor to all sphingolipid classes, is represented by only one major molecular species in animals
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)