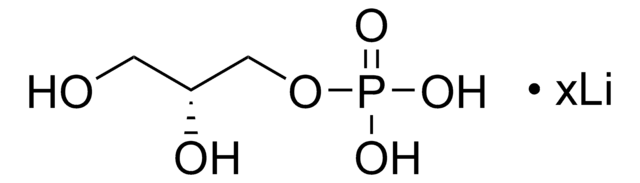
5.30655
Mitochondrial GPDH Inhibitor, iGP-1
別名:
Mitochondrial GPDH Inhibitor, iGP-1, N-(4-(1H-Benzoimidazol-2-yl)-phenyl)-succinamic acid, Mitochondrial sn-Glycerol-3-Phosphate Dehydrogenase Inhibitor, mGPDH Inhibitor, inhibitor of m GPDH 1, iGP1, N-(4-(1H-Benzoimidazol-2-yl)-phenyl)-succinamic acid, Mitochondrial sn-Glycerol-3-Phosphate Dehydrogenase Inhibitor, mGPDH Inhibitor, inhibitor of mGPDH 1, iGP1
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
おすすめの製品
アッセイ
≥98% (HPLC)
品質水準
形状
solid
有効性
6.3 μM IC50
メーカー/製品名
Calbiochem®
保管条件
OK to freeze
protect from light
色
beige
溶解性
DMSO: 50 mg/mL
保管温度
2-8°C
詳細
A cell-permeable benzimidazolyl-phenylamidosuccinate compound that acts as a potent, selective, and dose-dependent inhibitor against mitochondrial, but not cytosolic, sn-glycerol-3-phosphate dehydrogenase (IC50 against mGPDH = 6.3 µM). Shown to directly interact with a single, allosteric binding site on mGPDH and displays mixed type of inhibition kinetics (Kic = 9.5 µM and Kiu = 14.6 µM, glycerol phosphate/GP-competitive against free and GP-uncompetitive against GP-bound mGPDH). Reduces H2O2 production by IQ site (IC50 = 13.6 µM) and inhibits ΔΨm driven by low concentrations of succinate (0.5 µM), but does not inhibit glutamate-, malate-, pyruvate-, or palmityoylcarnitine-driven ΔΨm. Does not prevent pyruvate uptake into cells or mitochondria and has no direct effect on Glycolysis in synaptosomes.
A cell-permeable thiadiazolyl-butyl-pyridazinyl compound that displays increased fluorescence with acidified pH (by 8-fold from pH 7.0 to 1.5; Ex 342 nm & Em 378 nm) and selectively inhibits mitochondrial sn-glycerol 3-phosphate (GP) dehydrogenase (mGPDH) activity (IC50 = 6.3 µM by DCPIP assay using rat skeletal muscle mitochondria preparation with 1 µ Ca2+), but not cytosolic GPDH (up to 80 µM using rabbit cGPDH), by targeting free mGPDH in a GP-competitive manner (KicKiu2-dependent complex I activity and little potency toward unbiquinol/QH2-dependent complex III activity, nor FMN-dependent complex I or II activity. Under conditions where Lactate dehydrogenase-mediated NAD+ generation is blocked by Oxamate in murine cortical synaptosome preparations, a small but significant dependence on mGPDH-mediated GP shuttle for NAD+ generation to support the high glycolytic demand for pyruvate generation from glucose (15 mM) is reported, as evidenced by a more pronounced mitochondrial respiration inhibition in the presence of FCCP and Oligomycin (4 µg/mL; Cat. No. 495455) with the combined treatment of 0.5 mM aminooxyacetate (AOA) and 100 µM iGP-1 than with AOA alone.
生物化学的/生理学的作用
Cell permeable: yes
Primary Target
mGPDH
mGPDH
包装
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
再構成
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
その他情報
Orr, A.L., et al. 2014. PLoS One9, e89938.
法的情報
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)