おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
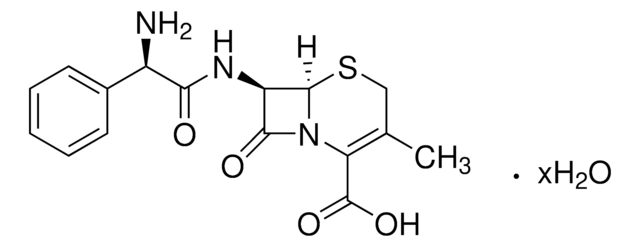
cephalexin
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
InChI
1S/C16H17N3O4S.H2O/c1-8-7-24-15-11(14(21)19(15)12(8)16(22)23)18-13(20)10(17)9-5-3-2-4-6-9;/h2-6,10-11,15H,7,17H2,1H3,(H,18,20)(H,22,23);1H2/t10-,11-,15-;/m1./s1
InChI Key
AVGYWQBCYZHHPN-CYJZLJNKSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Cephalexin monohydrate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Jin Yang et al.
Journal of hazardous materials, 360, 481-489 (2018-08-26)
Photocatalytic ozonation has great potential in wastewater treatment. However, the role of ozone and the contribution of photogenerated hole in this process have not been fully understood. Here three WO3 materials are synthesized and used as model catalysts in visible-light
Daniela Peneda Pacheco et al.
Journal of materials chemistry. B, 7(32), 4940-4952 (2019-08-15)
Mucus is a natural barrier with a protective role that hinders drug diffusion, representing a steric and interactive barrier to overcome for an effective drug delivery to target sites. In diseases like cystic fibrosis (CF), pulmonary mucus exhibits altered features
Ali Rayegan et al.
International journal of biological macromolecules, 113, 317-328 (2018-02-27)
A novel drug delivery system, loaded the drug cephalexin on the basil seed mucilage coated magnetic nanoparticles (Fe3O4@BSM-CPX) was prepared and characterized by means of X-ray diffraction (XRD), Furier Transform Infrared (FTIR), Field Emission Scanning Electron Microscope (FESEM), Vibrating Sample
Gerlinde F Plöger et al.
Journal of pharmaceutical sciences, 109(6), 1846-1862 (2020-04-03)
Literature data and results of experimental studies relevant to the decision to allow waiver of bioequivalence studies in humans for the approval of immediate release solid oral dosage forms containing cephalexin monohydrate are presented. Solubility studies were performed in accordance
C W Derrick et al.
Postgraduate medical journal, 59 Suppl 5, 43-46 (1983-01-01)
Cephalexin remains an effective and highly useful antibiotic for the treatment of streptococcal and staphylococcal skin infections. Twelve years of experience have not diminished its efficacy, and cure rates of 90% or higher continue to be achieved. Its resistance to
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)