すべての画像(1)
About This Item
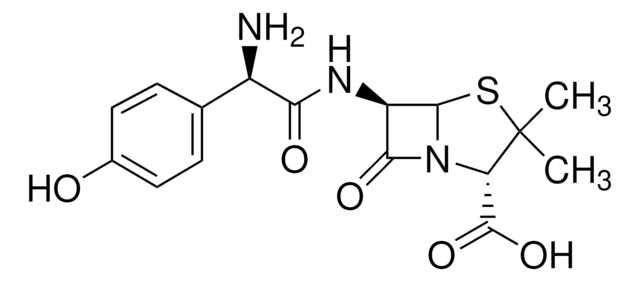
実験式(ヒル表記法):
C16H19N3O5S · 3H2O
CAS番号:
分子量:
419.45
Beilstein:
7507120
MDL番号:
UNSPSCコード:
41116107
PubChem Substance ID:
NACRES:
NA.24
おすすめの製品
グレード
pharmaceutical primary standard
認証
EP
APIファミリー
amoxicillin
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
O.O.O.CC1(C)S[C@@H]2[C@H](NC(=O)[C@H](N)c3ccc(O)cc3)C(=O)N2[C@H]1C(O)=O
InChI
1S/C16H19N3O5S.3H2O/c1-16(2)11(15(23)24)19-13(22)10(14(19)25-16)18-12(21)9(17)7-3-5-8(20)6-4-7;;;/h3-6,9-11,14,20H,17H2,1-2H3,(H,18,21)(H,23,24);3*1H2/t9-,10-,11+,14-;;;/m1.../s1
InChI Key
MQXQVCLAUDMCEF-CWLIKTDRSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
アプリケーション
Amoxicillin trihydrate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Resp. Sens. 1 - Skin Sens. 1
保管分類コード
11 - Combustible Solids
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
A0800000-100MG:
A0800000:
A0800000-1EA:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
Katherine E Fleming-Dutra et al.
Pediatrics, 134(6), 1059-1066 (2014-11-19)
Previous research suggests that physicians may be less likely to diagnose otitis media (OM) and to prescribe broad-spectrum antibiotics for black versus nonblack children. Our objective was to determine whether race is associated with differences in OM diagnosis and antibiotic
Ambrose Agweyu et al.
Tropical medicine & international health : TM & IH, 19(11), 1310-1320 (2014-08-19)
To determine the extent and pattern of treatment failure (TF) among children hospitalised with community-acquired pneumonia at a large tertiary hospital in Kenya. We followed up children aged 2-59 months with WHO-defined severe pneumonia (SP) and very severe pneumonia (VSP)
In acute calculous cholecystitis, antibiotics after cholecystectomy did not reduce infection.
Daniel I Steinberg
Annals of internal medicine, 161(12), JC4-JC4 (2014-12-17)
Gilson C N Franco et al.
International journal of clinical pharmacology and therapeutics, 52(5), 425-430 (2014-04-24)
To compare the pharmacokinetic profiles and to evaluate the bioequivalence of two commercial amoxicillin suspension formulations (500 mg/5 mL AMOXIL®, reference formulation and AMOXI-PED®, test formulation) in healthy Brazilian volunteers. Under fasting condition, 25 volunteers (13 males and 12 females)
Jean Marc Regimbeau et al.
JAMA, 312(2), 145-154 (2014-07-10)
Ninety percent of cases of acute calculous cholecystitis are of mild (grade I) or moderate (grade II) severity. Although the preoperative and intraoperative antibiotic management of acute calculous cholecystitis has been standardized, few data exist on the utility of postoperative
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)