おすすめの製品
グレード
certified reference material
pharmaceutical secondary standard
品質水準
認証
traceable to Ph. Eur. B1110000
APIファミリー
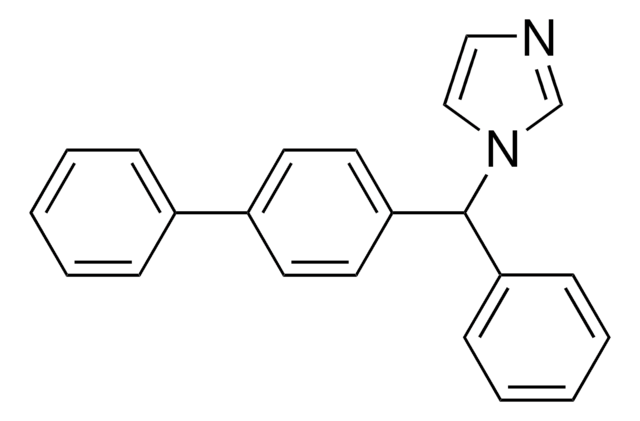
bifonazole
CofA
current certificate can be downloaded
テクニック
HPLC: suitable
gas chromatography (GC): suitable
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
c1ccc(cc1)C(c2ccc(cc2)-c3ccccc3)n4ccnc4
InChI
1S/C22H18N2/c1-3-7-18(8-4-1)19-11-13-21(14-12-19)22(24-16-15-23-17-24)20-9-5-2-6-10-20/h1-17,22H
InChI Key
OCAPBUJLXMYKEJ-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Bifonazole is a substituted imidazole analog with therapeutic potential in treating invasive mucosal infections. It exhibits antifungal activity by suppressing the proliferation of dermatophytes, yeasts and fungi affecting the skin and nails. Its mode of action involves blocking the fungal ergosterol biosynthetic pathway and additional inhibition of terpenoid biosynthesis.
Bifonazole is a substituted imidazole analog with therapeutic potential in treating invasive mucosal infections. It exhibits antifungal activity by suppressing the proliferation of dermatophytes, yeasts and fungi affecting the skin and nails. Its mode of action involves blocking the fungal ergosterol biosynthetic pathway and additional inhibition of terpenoid biosynthesis.
アプリケーション
Bifonazole may be used as a pharmaceutical reference standard for the quantification of the analyte in pharmaceutical formulations using spectrophotometric technique.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
生物化学的/生理学的作用
Bifonazole is an imidazole-based anti-fungal agent with broad spectrum activity against many fungi, molds, yeast and some Gram-positive bacteria. Bifonazole inhibits ergosterol biosynthetic protein 28 and Cytochrome P450 2B4.
アナリシスノート
このような2次標準は、USP、EP(PhEur)、BPの1次標準にマルチトレーサビリティを提供します。
その他情報
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
脚注
To see an example of a Certificate of Analysis for this material enter LRAC3256 in the slot below. This is an example certificate only and may not be the lot that you receive.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral
保管分類コード
11 - Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR1296-1G:
PHR1296-1G-PW:
PHR1296-1ML-PW:
Validated spectrophotometric methods for the determination of bifonazole in pharmaceuticals by charge transfer complexation
Ismail SBN, et al.
Journal of the Association of Arab Universities for Basic and Applied Sciences, 19(1), 8-14 (2016)
Determination of bifonazole in creams containing methyl-and propyl p-hydroxybenzoate by derivative spectrophotometric method
Popovic G, et al.
Journal of Pharmaceutical and Biomedical Analysis, 33(1), 131-136 (2003)
Yonghong Zhao et al.
The Journal of biological chemistry, 281(9), 5973-5981 (2005-12-24)
To better understand ligand-induced structural transitions in cytochrome P450 2B4, protein-ligand interactions were investigated using a bulky inhibitor. Bifonazole, a broad spectrum antifungal agent, inhibits monooxygenase activity and induces a type II binding spectrum in 2B4dH(H226Y), a modified enzyme previously
Di-Qing Luo et al.
Mycoses, 54(6), e780-e784 (2011-05-28)
Interdigital ulcer is an exceptionally rare condition while erosio interdigitalis blastomycetica is common for candidiasis. Four Chinese patients with Candida interdigital ulcers were reported. The exudates were examined directly and cultured for fungi. Skin biopsies were stained with haematoxylin-eosin and
Nehama Linder et al.
Birth defects research. Part A, Clinical and molecular teratology, 88(3), 201-204 (2009-12-17)
Neonatal limb reduction defects may be caused by exposure to an external agent. The azole derivatives are used in the treatment of systemic and dermal mycoses. Their relative teratogenic risk is still controversial. We describe two newborns with severe limb
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)