おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
形状
powder
保管条件
desiccated
色
white to brown
溶解性
DMSO: 10 mg/mL, clear
保管温度
−20°C
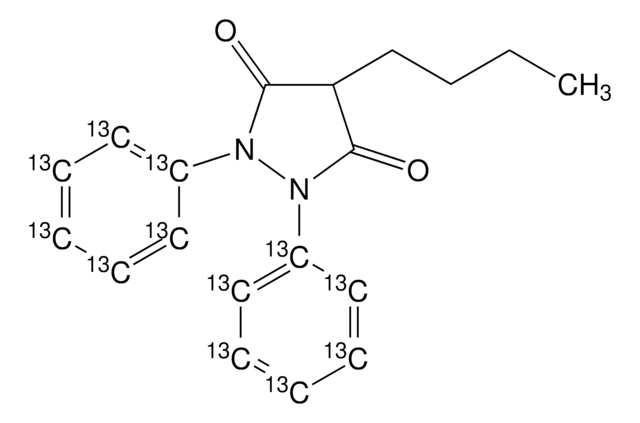
InChI
1S/C19H20N2O3/c1-2-3-9-17-18(23)20(14-7-5-4-6-8-14)21(19(17)24)15-10-12-16(22)13-11-15/h4-8,10-13,17,22H,2-3,9H2,1H3
InChI Key
HFHZKZSRXITVMK-UHFFFAOYSA-N
詳細
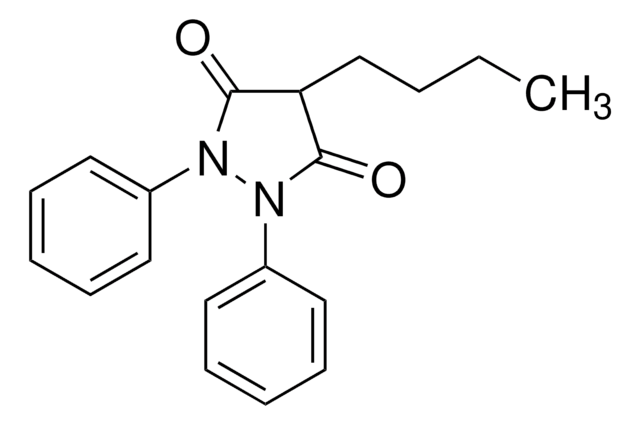
Oxyphenbutazone is a derivative compound of phenylbutazone.
生物化学的/生理学的作用
Oxyphenbutazone is a non-steroid anti inflammatory; anti Mycobacterium tuberculosis agent. Oxyphenbutazone is known to cause inflammatory effects on tissues. Oxyphenbutazone, as a drug, decreases cellular exudates, without involving the pituitary-adrenal axis or the immunity response. Though the drug delivers a number of side effects, it is considered to be less toxic than phenylbutazone, due to decreased rate of intestinal absorption.
Oxyphenbutazone is an NSAID that has been shown to preferentially kill non-replicating Mycobaterium tuberculosis maintained in media that simulates the mildly acidic, in vivo conditions where drug-resistant, non replicating subpopulations of the bacteria reside in hosts. The compound has little or no affect on replication M. tuberculosis grown in normal liquid cultures.
その他情報
Air sensitive
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Aquatic Acute 1
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SML0540-50MG:
SML0540-VAR:
SML0540-10MG:
SML0540-BULK:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
A Kadir et al.
Journal of veterinary pharmacology and therapeutics, 20(1), 54-60 (1997-02-01)
Phenylbutazone was administered intravenously and intramuscularly at a dosage rate of 4.4 mg/kg to a group of 6 female camels in a two-period crossover study. After intravenous (i.v.) administration, disposition was characterised by a two-compartment open model, with a low
Ulcer of the cecum during oxyphenbutazone (tandearil) therapy.
Debenham G P
Canadian Medical Association Journal, 94(22), 1182-1182 (1966)
M D Veiga et al.
Journal of pharmaceutical and biomedical analysis, 28(5), 973-982 (2002-06-01)
The interactions between a nonsteroidal anti-inflammatory drugs, oxyphenbutazone (OPB), with two cyclodextrins, beta-cyclodextrin (beta-CD) and gamma-cyclodextrin (gamma-CD), have been studied in an aqueous medium and in the solid state. Differential scanning calorimetry, hot stage microscopy, thermogravimetric analysis and X-ray diffraction
B Razdan et al.
Drug development and industrial pharmacy, 25(9), 1051-1056 (1999-10-13)
Dissolution-dialysis studies of commercial tablets of oxyphenbutazone were carried out to establish the applicability of this technique for the in vitro evaluation of oxyphenbutazone dosage form. While disintegration time and dissolution rate studies did not give a true indication of
N S Matthews et al.
American journal of veterinary research, 62(5), 673-675 (2001-05-09)
To describe the pharmacokinetics of phenylbutazone and oxyphenbutazone after IV administration in miniature donkeys. 6 clinically normal miniature donkeys. Blood samples were collected before and 5, 10, 20, 30, 45, 60, 90, 120, 180, 240, 300, 360, and 480 minutes
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)