おすすめの製品
詳細
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
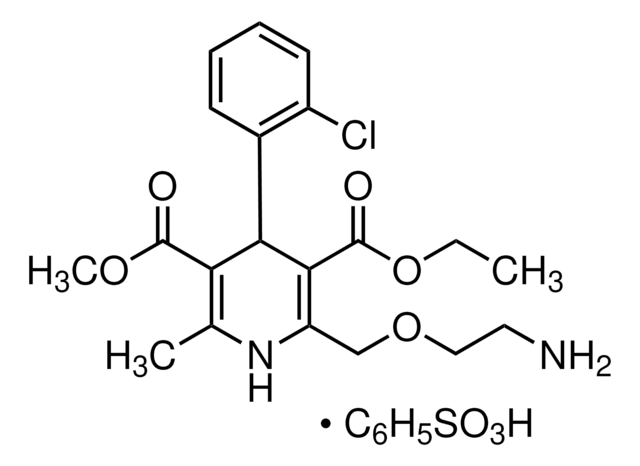
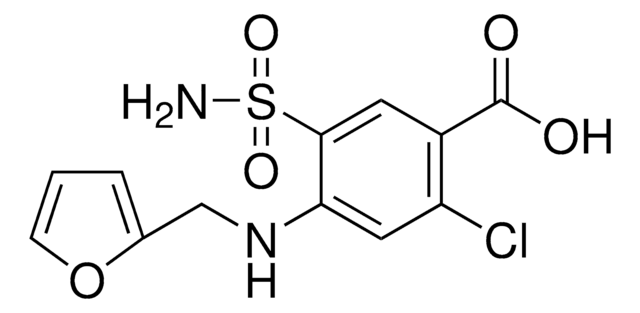
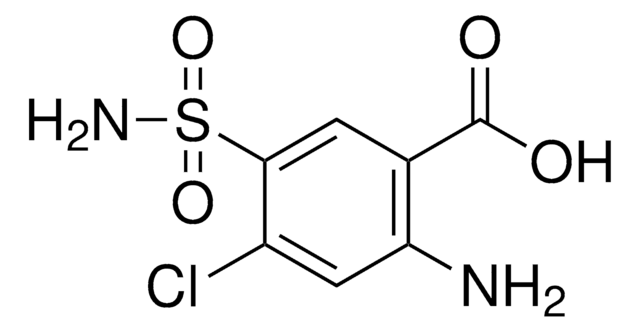
It is an impurity of the long-acting calcium channel blocker, amlodipine, that belongs to the class of 1,4-Dihydropyridine (DHP) calcium antagonists. The parent active pharmaceutical ingredient is used in the treatment of hypertension and anginal chest pain.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is an impurity of the long-acting calcium channel blocker, amlodipine, that belongs to the class of 1,4-Dihydropyridine (DHP) calcium antagonists. The parent active pharmaceutical ingredient is used in the treatment of hypertension and anginal chest pain.
アプリケーション
This pharmaceutical secondary standard can also be used as follows:
- Development of a stability-indicating reverse-phase ultra-performance liquid chromatographic (RP-UPLC) method for determining related impurities of S(−)amlodipine and S(−)metoprolol succinate in their combined tablet dosage
- Ultra-high pressure liquid chromatographic (UHPLC) separation and estimation of amlodipine and bisoprolol-related impurities in the combined pharmaceutical formulation of the parent APIs
- Development and validation of a UHPLC method for quantifying impurities of valsartan, amlodipine besylate, and hydrochlorothiazide in their combined tablet dosage
- Separation and determination of amlodipine and atorvastatin, along with their impurities using a stability-indicating RP-HPLC method in their combined solid dosage forms
アナリシスノート
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
脚注
To see an example of a Certificate of Analysis for this material enter LRAB9841 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
おすすめ製品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
関連製品
製品番号
詳細
価格
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - STOT RE 2
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR2050-50MG:
この製品を見ている人はこちらもチェック
Robust UHPLC separation method development for multi-API product containing amlodipine and bisoprolol: the impact of column selection
Kormany, R, et al.
Chromatographia, 77, 1119-1127 (2014)
Stability-indicating method for the determination of assay and quantification of impurities in amlodipine-atorvastatin combination dosage form by RP-HPLC
Sangeetha D and Vadlamudi MK
Journal of Liquid Chromatography and Related Technologies, 40, 576-598 (2017)
Development and Validation of Stability-Indicating RP-UPLC Method for Simultaneous Determination of Related Substances of S (-) Amlodipine and S (-) Metoprolol Succinate in Fixed Dose Combination Tablet Dosage Form
Shitole S, et al.
Chromatography Research International, 2014 (2014)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)