Y0000412
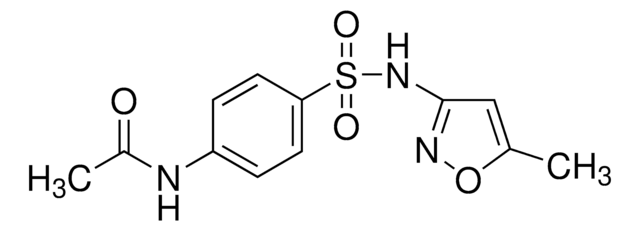
Sulfamethoxazole impurity A
European Pharmacopoeia (EP) Reference Standard
別名:
N4-Acetylsulfamethoxazole, N-Acetyl sulfamethoxazole, Acetyl-sulfamethoxazole, N-{4-{[(5-Methyl-3-isoxazolyl)amino]sulfonyl}phenyl}acetamide, Sulfisomezole-N4-acetate
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
実験式(ヒル表記法):
C12H13N3O4S
CAS番号:
分子量:
295.31
Beilstein:
285801
MDL番号:
UNSPSCコード:
41116107
PubChem Substance ID:
NACRES:
NA.24
おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
sulfamethoxazole
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
O=S(NC1=NOC(C)=C1)(C2=CC=C(NC(C)=O)C=C2)=O
InChI
1S/C12H13N3O4S/c1-8-7-12(14-19-8)15-20(17,18)11-5-3-10(4-6-11)13-9(2)16/h3-7H,1-2H3,(H,13,16)(H,14,15)
InChI Key
GXPIUNZCALHVBA-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Sulfamethoxazole impurity A EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
O Spreux-Varoquaux et al.
Journal of chromatography, 274, 187-199 (1983-05-13)
A normal-phase high-performance liquid chromatographic method was developed to determine therapeutic concentrations of trimethoprim, sulphamethoxazole, and its N4-acetyl derivative in biological fluids. The compounds are extracted at pH 6.2 using ethyl acetate--chloroform in a single extraction. The detection limit is
T B Vree et al.
The Veterinary quarterly, 9(4), 378-381 (1987-10-01)
The snail Cepaea hortensis is able to acetylate and hydroxylate sulphamethoxazole in a similar way to man. About one percent deacetylation, but no hydroxylation of N4-acetysulphamethoxazole takes place. The relative rates of the metabolic pathways for hydroxylation and acetylation of
Y Zhang et al.
Analytical biochemistry, 212(2), 481-486 (1993-08-01)
We present gas chromatographic-mass spectrometric assays for (i) the concentration of sulfamethoxazole and (ii) the concentration and molar percentage enrichment of acetyl-sulfamethoxazole in biological fluids. The compounds are extracted with ethyl acetate, derivatized with either diazomethane or pentafluorobenzyl bromide, and
A Weber et al.
Journal of chromatography, 278(2), 337-345 (1983-12-09)
We describe a rapid, precise and simple procedure for the quantitative determination of trimethoprim, sulfamethoxazole, and N4-acetylsulfamethoxazole in body fluids by reversed-phase high-performance liquid chromatography. This method utilizes antipyrine as an internal standard with the compounds detected by dual-wavelength monitoring
T B Vree et al.
The Veterinary quarterly, 9(4), 381-384 (1987-10-01)
The turtle Pseudemys scripta elegans is able to hydroxylate and acetylate Sulphamethoxazole in a way that is comparable to man, i.e. the rate and yield of hydroxylation equals that of the acetylation. The hydroxy metabolites 5-hydroxy- and N4-acetyl-5-hydroxysulphamethoxazole are not
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)