06892
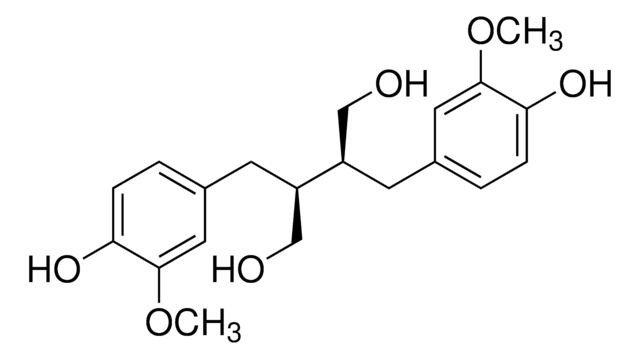
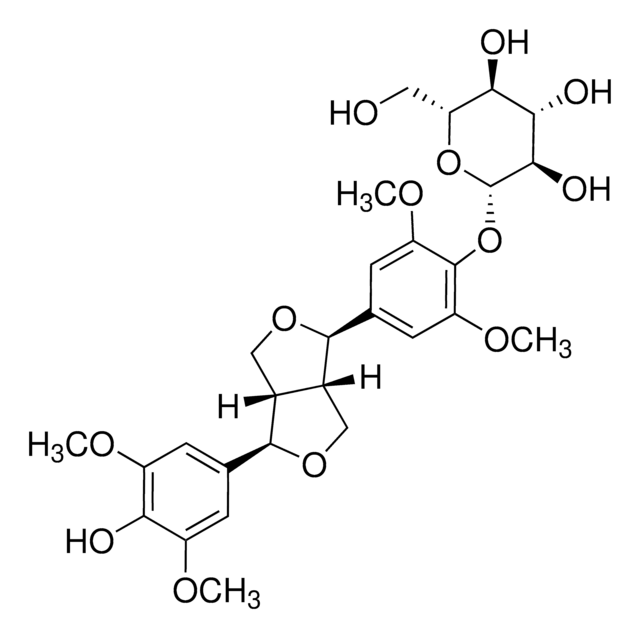
(+)-ラリシレシノール
≥95.0% (HPLC)
別名:
(2S,3R,4R)-テトラヒドロ-2-(4-ヒドロキシ-3-メトキシフェニル)-4-[(4-ヒドロキシ-3-メトキシフェニル)メチル]-3-フランメタノール, 4-[(2S,3R,4R)-4-[(4-ヒドロキシ-3-メトキシフェニル)メチル]-3-(ヒドロキシメチル)オキソラン-2-イル]-2-メトキシフェノール, NSC 329247
About This Item
おすすめの製品
品質水準
アッセイ
≥95.0% (HPLC)
フォーム
powder
アプリケーション
metabolomics
vitamins, nutraceuticals, and natural products
SMILES記法
COc1cc(C[C@H]2CO[C@@H]([C@H]2CO)c3ccc(O)c(OC)c3)ccc1O
COc1cc(C[C@H]2CO[C@@H]([C@H]2CO)c3ccc(O)c(OC)c3)ccc1O
InChI
1S/C20H24O6/c1-24-18-8-12(3-5-16(18)22)7-14-11-26-20(15(14)10-21)13-4-6-17(23)19(9-13)25-2/h3-6,8-9,14-15,20-23H,7,10-11H2,1-2H3/t14-,15-,20+/m0/s1
InChI Key
MHXCIKYXNYCMHY-AUSJPIAWSA-N
詳細
アプリケーション
- in the purification, identification, and analysis of lignan phytoestrogens and lignan glycosides
- for the determination of the enantiomeric composition of lariciresinol from the pinoresinol to lariciresinol reduction reaction using high-performance liquid chromatography (HPLC)
- forqualitative and quantitative analysis of lignans in seven types of triticalegrain using ultra-performance liquid chromatography (UPLC)
生物化学的/生理学的作用
包装
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Aquatic Acute 1
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
06892-BULK:
06892-VAR:
06892-10MG:
この製品を見ている人はこちらもチェック
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)