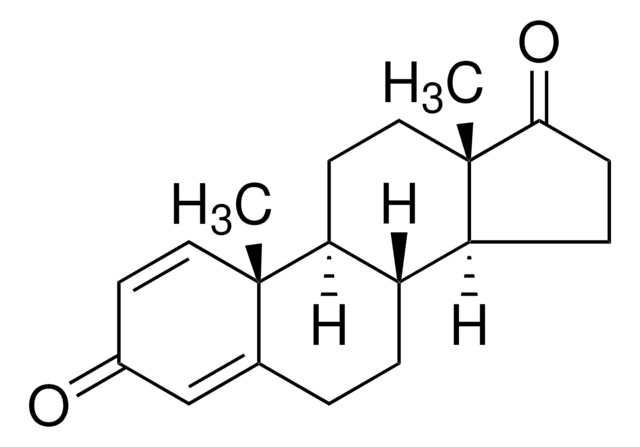
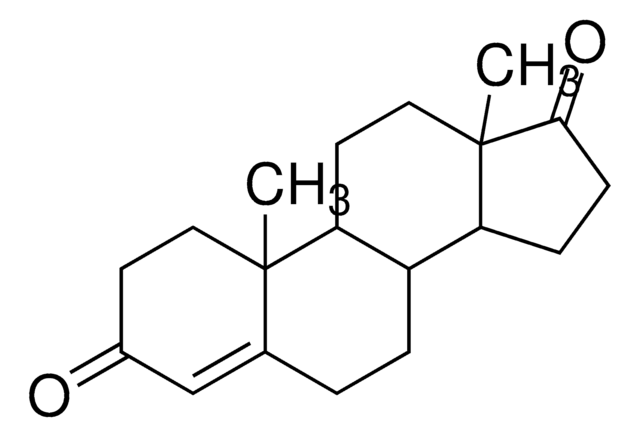
おすすめの製品
由来生物
synthetic (organic)
品質水準
アッセイ
≥85% (HPLC)
フォーム
powder
薬剤管理
USDEA Schedule IIIN; regulated under CDSA - not available from Sigma-Aldrich Canada
溶解性
chloroform: 50 mg/mL, clear, colorless to faintly yellow
保管温度
room temp
SMILES記法
[H][C@@]12CCC3=CC(=O)C=C[C@]3(C)[C@@]1([H])CC[C@]4(C)C(=O)CC[C@@]24[H]
InChI
1S/C19H24O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h7,9,11,14-16H,3-6,8,10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1
InChI Key
LUJVUUWNAPIQQI-QAGGRKNESA-N
遺伝子情報
human ... CYP19A1(1588)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
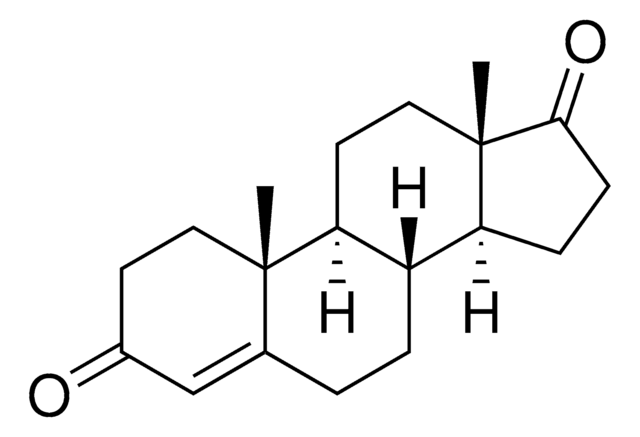
1,4-Androstadiene-3,17-dione is a 17-keto anabolic steroid.
生物化学的/生理学的作用
1,4-Androstadiene-3,17-dione is a prohormone that converts to an active steroid through the 17bHSD enzyme. 1,4-Androstadiene-3,17-dione is a metabolite of progesterone.
1,4-Androstadiene-3,17-dione is useful in forming pharmaceutically important steroids. Regio- and stereospecific hydroxylation of 1,4-androstadiene-3,17-dione increases its biological activity. Its derivative is also used to produce high-value bile acids. 1,4-Androstadiene-3,17-dione serves as a precursor for the synthesis of progesterone, testosterone, cortisol, estradiol, cortisone, prednisolone, and prednisone.
この製品を見ている人はこちらもチェック
Rituraj Batth et al.
Plants (Basel, Switzerland), 9(9) (2020-09-10)
Steroids are a group of organic compounds that include sex hormones, adrenal cortical hormones, sterols, and phytosterols. In mammals, steroid biosynthesis starts from cholesterol via multiple steps to the final steroid and occurs in the gonads, adrenal glands, and placenta.
Wei Wei et al.
Applied and environmental microbiology, 76(13), 4578-4582 (2010-05-11)
3-Ketosteroid-Delta(1)-dehydrogenase, KsdD(M), was identified by targeted gene disruption and augmentation from Mycobacterium neoaurum NwIB-01, a newly isolated strain. The difficulty of separating 4-androstene-3,17-dione (AD) from 1,4-androstadiene-3,17-dione (ADD) is a key bottleneck to the microbial transformation of phytosterols in industry. This
Victoria Giorgi et al.
World journal of microbiology & biotechnology, 35(1), 12-12 (2019-01-04)
Microorganisms were isolated from industrial wool scouring effluents and from the soil adjacent to the wastewater treatment lagoon, both sterols-rich environments, in order to search for novel biocatalysts able to transform cholesterol. The isolates were identified on the basis of
M V Donova et al.
Microbiology (Reading, England), 153(Pt 6), 1981-1992 (2007-05-29)
Modified beta-cyclodextrins have been shown previously to enhance sterol conversion to 4-androstene-3,17-dione (AD) and 1,4-androstadiene-3,17-dione (ADD) by growing Mycobacterium spp. The enhancement effect was mainly attributed to steroid solubilization by the formation of inclusion complexes with modified cyclodextrins. In this
K Verheyden et al.
Analytica chimica acta, 586(1-2), 163-170 (2007-03-28)
Current evidence suggests that neo formation of the anabolic steroid boldenone (androsta-1,4-diene-17-ol-3-one) occurs in calves' faecal material, making it difficult to distinguish between illegally administered boldenone and its potential endogenous presence. This strengthens the urgent need to elucidate the pathway
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)