すべての画像(1)
About This Item
実験式(ヒル表記法):
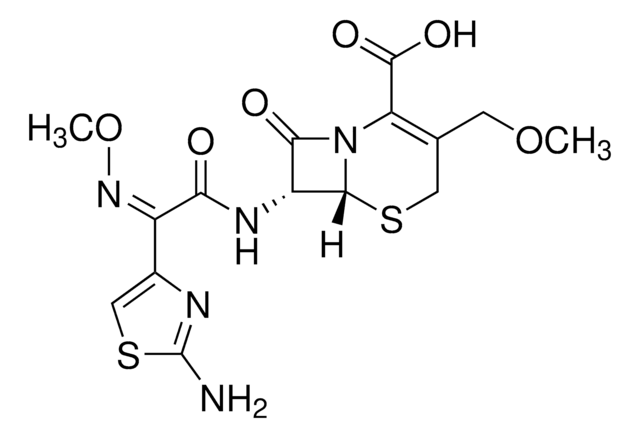
C21H33N3O5S
CAS番号:
分子量:
439.57
EC Number:
MDL番号:
UNSPSCコード:
51111800
PubChem Substance ID:
NACRES:
NA.77
おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
フォーム
powder
光学活性
[α]/D +215 to +245°, c = 1 in ethanol
色
white to beige
溶解性
DMSO: 5 mg/mL, clear (warmed)
保管温度
−20°C
SMILES記法
CC1(C)[C@H](C(OCOC(C(C)(C)C)=O)=O)N2C([C@](/N=C/N3CCCCCC3)([H])[C@@]2([H])S1)=O
InChI
1S/C21H33N3O5S/c1-20(2,3)19(27)29-13-28-18(26)15-21(4,5)30-17-14(16(25)24(15)17)22-12-23-10-8-6-7-9-11-23/h12,14-15,17H,6-11,13H2,1-5H3/b22-12+/t14-,15+,17-/m1/s1
InChI Key
NPGNOVNWUSPMDP-UTEPHESZSA-N
アプリケーション
Pivmecillinam has been used to test its efficacy as a breast cancer stem cells (CSCs) targeting agent.
生物化学的/生理学的作用
Pivmecillinam is a β-lactam antibiotic and a pivaloyl-containing pro-drug. It belongs to the class of amidinopenicillin. Pivmecillinam is an oral anti-microbial agent that can be considered for treating E. coli urinary tract infection (UTI).
Pivmecillinam is a gram negative antibiotic, and inhibitor of penicillin-binding protein 2 (PBP2).
Pivmecillinam is a gram negative antibiotic, and inhibitor of penicillin-binding protein 2 (PBP2). Pivmecillinam has been shown to have synergistic effects with several antibiotics including novobiocin and rifampin in gram negative bacteria.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SML0817-10MG:
SML0817-BULK:
SML0817-VAR:
SML0817-50MG:
最新バージョンのいずれかを選択してください:
試験成績書(COA)
Lot/Batch Number
Mette Vinther Skriver et al.
Scandinavian journal of infectious diseases, 36(10), 733-737 (2004-10-30)
A previous study unexpectedly showed an increased, statistically imprecise, risk of low Apgar score in children of women redeeming prescriptions for pivmecillinam in late pregnancy. To improve statistical precision we extended the previous dataset with data for 5 more y
Lindsay E Nicolle et al.
Scandinavian journal of infectious diseases, 39(8), 748-749 (2007-07-27)
Oral therapy options for pyelonephritis caused by ESBL producing E. coli are limited. We describe a woman with relapsing pyelonephritis due to a CTX-M ESBL E. coli who was cured with a prolonged course of pivmecillinam. This suggests pivmecillinam may
Birgit Stattin Norinder et al.
Antimicrobial agents and chemotherapy, 50(4), 1528-1530 (2006-03-30)
Similar changes in the periurethral and vaginal microflora were observed in 19 women with recurrent urinary tract infection following treatment with norfloxacin (NOR) or pivmecillinam (PIV). Escherichia coli strains were suppressed by both treatments. Staphylococcus spp. and enterococci colony counts
Beatrix S Traa et al.
International journal of epidemiology, 39 Suppl 1, i70-i74 (2010-04-02)
Ciprofloxacin, ceftriaxone and pivmecillinam are the antibiotics currently recommended by the World Health Organization (WHO) for the treatment of dysentery in children; yet there have been no reviews of the clinical effectiveness of these antibiotics in recent years. We reviewed
Miika Bergman et al.
Antimicrobial agents and chemotherapy, 53(3), 912-917 (2008-12-24)
During a 9-year study period from 1997 through 2005, the association between antimicrobial resistance rates in Escherichia coli and outpatient antimicrobial consumption was investigated in 20 hospital districts in Finland. A total of 754,293 E. coli isolates, mainly from urine
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)