すべての画像(1)
About This Item
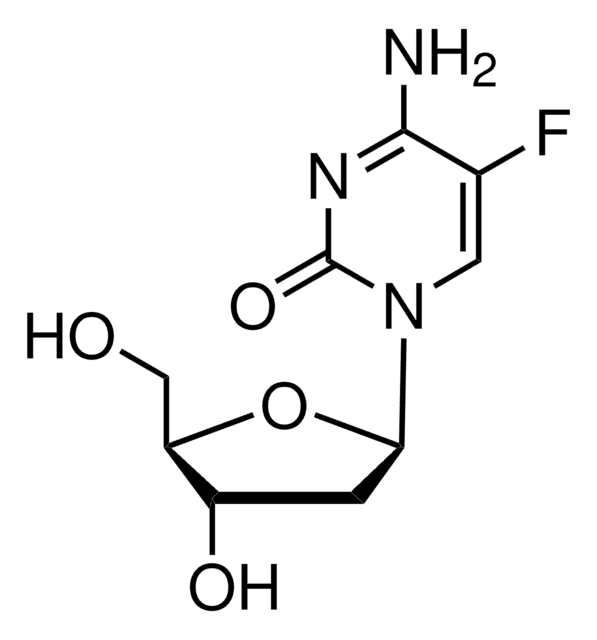
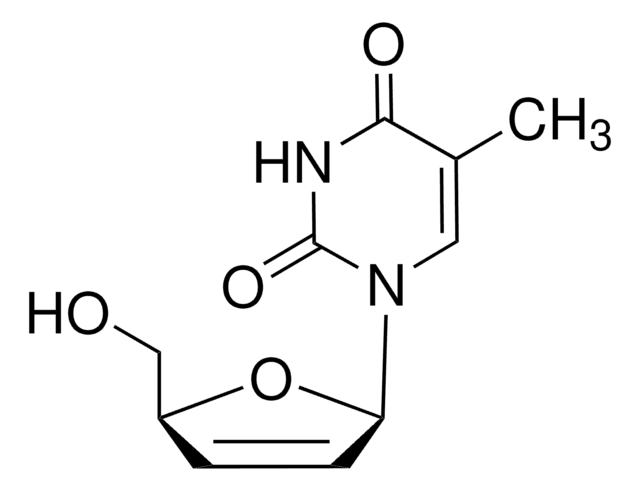
実験式(ヒル表記法):
C8H9FN2O3
CAS番号:
分子量:
200.17
EC Number:
MDL番号:
UNSPSCコード:
12352200
PubChem Substance ID:
NACRES:
NA.77
おすすめの製品
アプリケーション
Tegafur has been used as an internal standard for the analysis of 5′DFCR (5′deoxyfluorocytidine) and 5′DFUR (5′deoxyfluorouridine) using HPLC techniques. Tegafur has also been used as an internal standard for the chromatographic assay of azidothymidine (AZT).
生物化学的/生理学的作用
テガフールは、5-フルオロウラシルのプロドラッグであり、抗悪性腫瘍薬として用いられる代謝拮抗薬です。治療として切除された結腸直腸癌療法用のアジュバント化学療法として用いられています。
調製ノート
Tegafur is soluble in DMSO at a concentration that is greater than 50 mg/ml.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
T7205-25MG:
T7205-VAR:
T7205-BULK:
T7205-5MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Li-Feng Liu et al.
Chinese medical journal, 125(11), 1931-1935 (2012-08-14)
The pharmacokinetics of zidovudine (AZT) are possibly influenced by weight, age, sex, liver and renal functions, severity of disease, and ethnicity. Currently, little information is available on the steady-state pharmacokinetics of AZT in Chinese HIV-infected patients. The current study aimed
Muhammad Wasif Saif et al.
Expert opinion on investigational drugs, 18(3), 335-348 (2009-02-27)
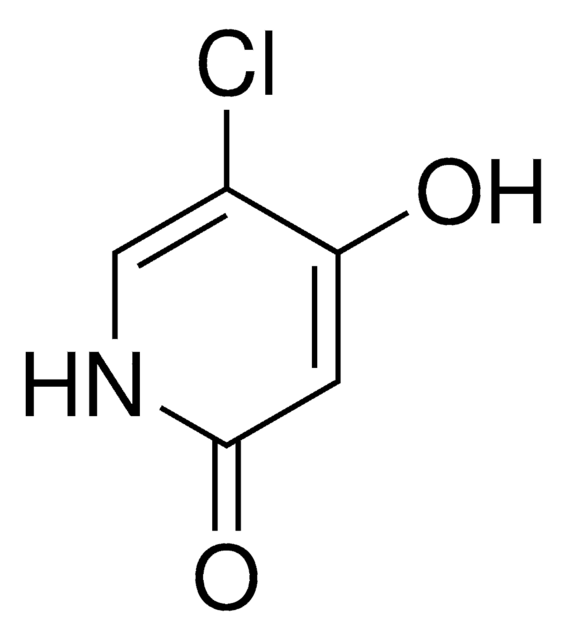
S-1 is an oral fluoropyrimidine that is designed to improve the antitumor activity of 5-fluorouracil (5-FU) concomitantly with an intent to reduce its toxicity. S-1 consists of tegafur, a prodrug of 5-FU combined with two 5-FU biochemical modulators:5-chloro-2,4-dihydroxypyridine (gimeracil or
Terence C Tang et al.
Neoplasia (New York, N.Y.), 12(3), 264-274 (2010-03-18)
Hepatocellular carcinoma (HCC) is an intrinsically chemotherapy refractory malignancy. Development of effective therapeutic regimens would be facilitated by improved preclinical HCC models. Currently, most models consist of subcutaneous human tumor transplants in immunodeficient mice; however, these do not reproduce the
Thierry Besnard et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 870(1), 117-120 (2008-06-20)
The 5FU prodrug capecitabine undergoes a 3-step enzymatic conversion, including the conversion of 5'DFRC into 5'DFUR by cytidine deaminase (CDA). The presence of CDA activity in blood led us to analyze the possible ex vivo conversion of 5'DFCR into 5'DFUR
Koji Oba
International journal of clinical oncology, 14(2), 85-89 (2009-04-25)
A consensus regarding standard adjuvant chemotherapy for curatively resected gastric cancer has not been obtained between Japan and the Western world. In order to evaluate the effect of a tegafur-based regimen (the most frequently used regimen in Japan) compared with
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)