추천 제품
Quality Level
분석
97%
양식
powder
mp
119-122 °C (lit.)
작용기
ketone
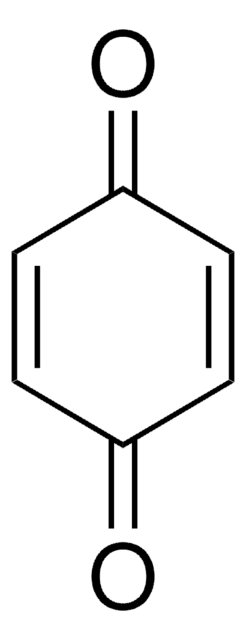
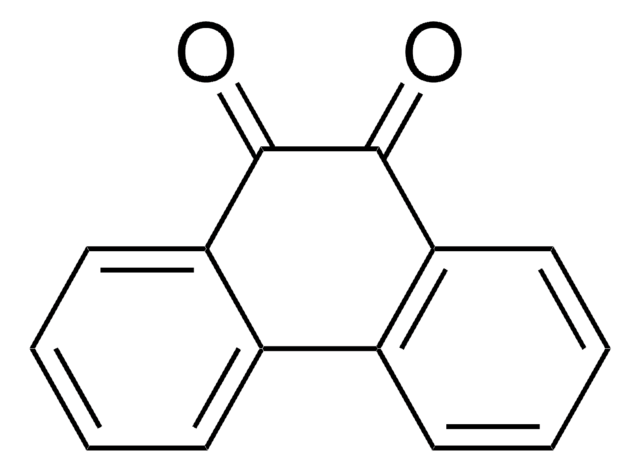
SMILES string
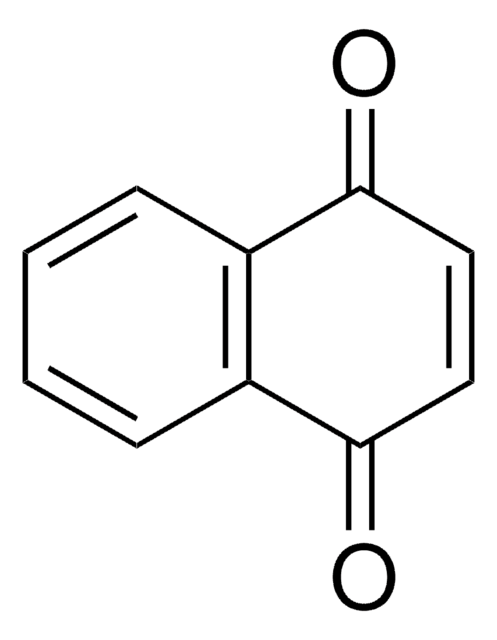
O=C1C=CC(=O)c2ccccc12
InChI
1S/C10H6O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6H
InChI key
FRASJONUBLZVQX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
1,4-Naphthoquinone was used as a potential inhibitor of monoamine oxidase and DNA topoisomerase activities. It was also used to inhibit the acetyltransferase activity.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1C - Skin Sens. 1 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
285.8 °F
Flash Point (°C)
141 °C
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Eduardo Coelho-Cerqueira et al.
Chemical biology & drug design, 83(4), 401-410 (2013-10-30)
Monoamine oxidase (MAO) action has been involved in the regulation of neurotransmitters levels, cell signaling, cellular growth, and differentiation as well as in the balance of the intracellular polyamine levels. Although so far obscure, MAO inhibitors are believed to have
Barbara Krajewska et al.
Bioorganic & medicinal chemistry, 15(12), 4144-4151 (2007-04-10)
In their inhibition-inducing interactions with enzymes, quinones primarily utilize two mechanisms, arylation and oxidation of enzyme thiol groups. In this work, we investigated the interactions of 1,4-naphthoquinone with urease in an effort to estimate the contribution of the two mechanisms
Daniela M Santos et al.
Molecular neurobiology, 47(1), 313-324 (2012-10-12)
Naphthoquinones are bioactive compounds widespread in nature that impact on several cellular pathways, including cell proliferation and survival, by acting as prooxidants and electrophiles. We have previously described the role of the synthetic isoxazole condensed 1,4-naphthoquinone derivative 1a in preventing
Paula F Carneiro et al.
Bioorganic & medicinal chemistry, 20(16), 4995-5000 (2012-07-17)
New oxirane derivatives were synthesized using six naphthoquinones as the starting materials. Our biological results showed that these oxiranes acted as trypanocidal agents against Trypanosoma cruzi with minimal cytotoxicity in the VERO cell line compared to naphthoquinones. In particular, oxirane
Don Antoine Lanfranchi et al.
Organic & biomolecular chemistry, 10(31), 6375-6387 (2012-07-11)
Improving the solubility of polysubstituted 1,4-naphthoquinone derivatives was achieved by introducing nitrogen in two different positions of the naphthoquinone core, at C-5 and at C-8 of menadione through a two-step, straightforward synthesis based on the regioselective hetero-Diels-Alder reaction. The antimalarial
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.