추천 제품
일반 설명
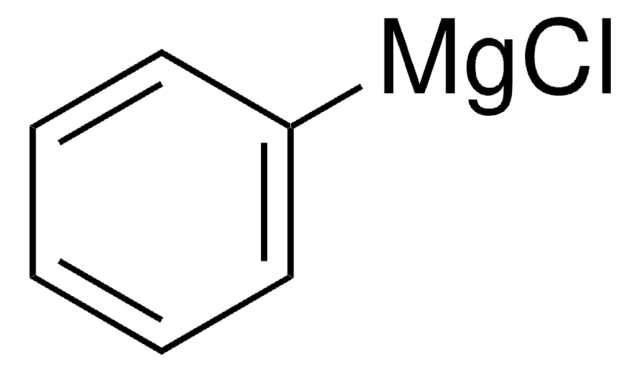
Phenylmagnesium bromide solution contains 3M phenylmagnesium bromide in diethyl ether. It can act as a strong acid and Lewis acid. It can undergo addition reaction with many unsaturated functional groups. The phenyl group can displace halide from other organic compounds. Phenylmagnesium bromide is a Grignard reagent. Reaction of β-cyclohexanedione (dihydroresorcinol) with phenylmagnesium bromide has been investigated.
애플리케이션
Phenylmagnesium bromide was used for the synthesis of end-functionalized regioregular poly(3-alkylthiophene)s. It was also used for the monoalkylation of aliphatic primary amine to generate secondary amines by the Grignard reaction of 1-[(alkylamino) methyl] benzotriazoles.
It may be used for synthesis of the following:
It may be used for synthesis of the following:
- 1,3,3-trimethyl-6-phenyl-2-oxabicyclo[2.2.2]octan-6-ol
- 6-benzyl-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-ol
- (3-(2-Dithiobenzoatepropionyl)propyl)dimethylmethoxysilane, reversible addition-fragmentation chain transfer polymerization (RAFT)-silane agent
- series of o-substituted benzophenones
포장
기타 정보
Storage below 25°C may cause formation of crystalline magnesium salts. Moving container to a warm location and occasional gentle swirling should redissolve the solid
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
표적 기관
Respiratory system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
-40.0 °F - closed cup
Flash Point (°C)
-40 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Synthesis of well-defined polymer brushes grafted onto silica nanoparticles via surface reversible addition-fragmentation chain transfer polymerization.
Li C and Benicewicz BC.
Macromolecules, 38(14), 5929-5936 (2005)
Synthesis of O-substituted benzophenones by Grignard reaction of 3-substituted isocoumarins.
Manivel P, et al.
Journal of the Chilean Chemical Society, 53(3), 1609-1610 (2008)
The reaction of beta-cyclohexanedione (dihydroresorcinol) and its ethyl enol ether with phenylmagnesium bromide.
G F WOODS et al.
Journal of the American Chemical Society, 70(6), 2174-2177 (1948-06-01)
S Schenone et al.
Farmaco (Societa chimica italiana : 1989), 45(12), 1309-1325 (1990-12-01)
The synthesis of 1,3,3-trimethyl-6-phenyl-2-oxabicyclo[2.2.2]octan-6-ol 2 and 6-benzyl-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-ol 3 starting from (+)-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-one and phenylmagnesium bromide or benzylmagnesium chloride, respectively, is described. Alcohols 2 and 3 gave a series of omega-dialkylaminoalkyl ethers 4 by reaction as sodium salts with omega-chloroalkyldialkylamines in toluene
Phenylmagnesium Bromide.
Richey HG, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2009)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.