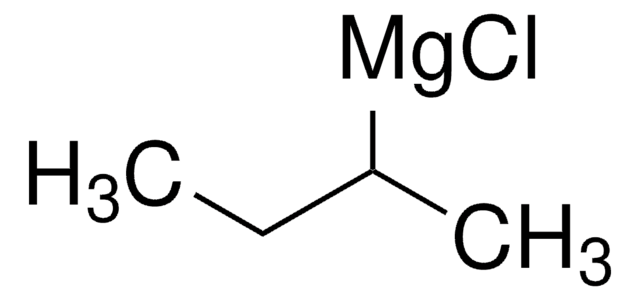
추천 제품
양식
liquid
Quality Level
반응 적합성
reaction type: Grignard Reaction
농도
2.0 M in diethyl ether
density
0.941 g/mL at 25 °C
SMILES string
CC(C)C[Mg]Br
InChI
1S/C4H9.BrH.Mg/c1-4(2)3;;/h4H,1H2,2-3H3;1H;/q;;+1/p-1
InChI key
CMWBEISSZHZIMU-UHFFFAOYSA-M
애플리케이션
Isobutylmagnesium bromide (iBuMgBr) is a general Grignard reagent used in the total synthesis of (+)-rishirilide B, glucolipsin A, and (+)-juvabione. It can also be used as a reagent in the synthesis of pyrrolidine-based influenza neuraminidase (NA) inhibitors.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 1 - Skin Irrit. 2 - STOT SE 3
표적 기관
Central nervous system
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point (°F)
-29.2 °F - closed cup
Flash Point (°C)
-34 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves
이미 열람한 고객
Enantio-and diastereocontrolled synthesis of (+)-juvabione employing organocatalytic desymmetrisation and photoinduced fragmentation.
Itagaki N and Iwabuchi Y
Chemical Communications (Cambridge, England), 69(11), 1175-1176 (2007)
Total synthesis of (+)-rishirilide B: Development and application of general processes for enantioselective oxidative dearomatization of resorcinol derivatives.
Mejorado LH and Pettus TRR
Journal of the American Chemical Society, 128(49), 15625-15631 (2006)
Structure-based characterization and optimization of novel hydrophobic binding interactions in a series of pyrrolidine influenza neuraminidase inhibitors.
Maring CJ, et al.
Journal of Medicinal Chemistry, 48(12), 3980-3990 (2005)
Structure assignment, total synthesis, and evaluation of the phosphatase modulating activity of glucolipsin A.
Furstner A, et al.
The Journal of Organic Chemistry, 69(2), 459-467 (2004)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.