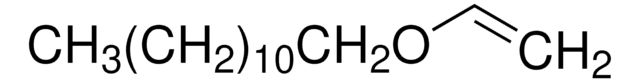모든 사진(1)
About This Item
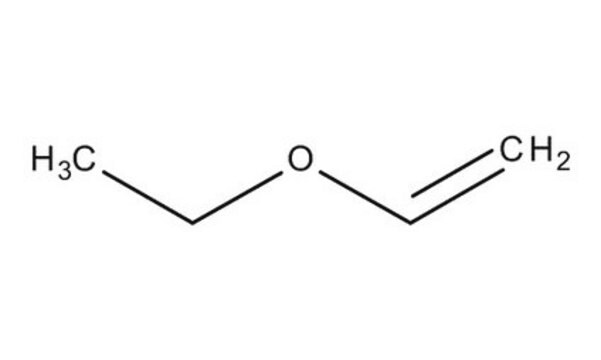
Linear Formula:
C2H5OCH=CH2
CAS Number:
Molecular Weight:
72.11
Beilstein:
605351
EC Number:
MDL number:
UNSPSC 코드:
12162002
eCl@ss:
39021025
PubChem Substance ID:
NACRES:
NA.23
추천 제품
Quality Level
분석
99%
형태
liquid
포함
0.1% KOH as stabilizer
0.1% potassium hydroxide as stabilizer
refractive index
n20/D 1.376 (lit.)
bp
33 °C (lit.)
mp
−116 °C (lit.)
density
0.753 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
CCOC=C
InChI
1S/C4H8O/c1-3-5-4-2/h3H,1,4H2,2H3
InChI key
FJKIXWOMBXYWOQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Ethyl vinyl ether, also known as ethoxy ethane is highly reactive due to the presence of the vinyl group and the ether functionality. It can polymerize via different methods like radical polymerization, cationic polymerization, oroxidative polymerization, yielding poly(ethyl vinyl ether) or PVE. It is widely used as a monomer to synthesize copolymers and block polymers with thermal stability and flexibility for various applications such as the synthesis of silicone hydrogel for contact lenses, tissue engineering, 3D bioprinting, drug delivery, and Li-ion batteries.
애플리케이션
Ethyl vinyl ether can be used as:
- A monomer to synthesize amphiphilic block copolymers to fabricate protein-repelling polymersomes to produce spherical nanoparticles. These nanoparticles can be used as drug carriers.
- A precursor to synthesize polymer electrolytes and cathode materials for solid-state lithium-ion batteries via UV photopolymerizations.
- A monomer to synthesize poly(vinyl ether)s with controlled molecular weight and narrow dispersity via photoinduced free radical-promoted cationic reversible addition–fragmentation chain transfer (RAFT) polymerization. These polymers are used to fabricate 3D objects with different thicknesses by employing stereolithography-based 3D printing.
- H-bonded Reusable Template Assisted para-Selective Ketonisation: This study discusses the use of ethyl vinyl ether in catalytic processes involving palladium and hexafluoroisopropanol to achieve para-selectivity in ketonisation, relevant for synthesizing complex organic compounds used in pharmaceuticals and materials science (A Maji, A Dahiya, G Lu, T Bhattacharya, 2018).
- Mechanistic Insight into Photocontrolled Cationic Polymerization: Explores the photocontrolled polymerization of ethyl vinyl ether, providing valuable knowledge for the development of light-responsive materials, which could have applications in drug delivery and smart material systems (Q Michaudel, T Chauviré, V Kottisch, 2017).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 3 - Flam. Liq. 2 - STOT SE 3
표적 기관
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
-49.0 °F
Flash Point (°C)
-45 °C
개인 보호 장비
Eyeshields, Faceshields, Gloves
이미 열람한 고객
A PROXYL-Type Norbornene Polymer for High-Voltage Cathodes in Lithium Batteries
Hatakeyama-Sato, et al.
Macromolecular Rapid Communications, 42, 2100374-2100374 (2021)
Polyglycidol-stabilized nanoparticles as a promising alternative to nanoparticle PEGylation: polymer synthesis and protein fouling considerations
Haiqin Du, et al.
Langmuir, 36, 1266-1278 (2020)
O Schalk et al.
The journal of physical chemistry. A, 119(45), 11105-11112 (2015-10-23)
A series of different alkyl vinyl ethers is investigated to decipher the possible reaction channels upon photoexcitation to the π3s-Rydberg and the ππ*-valence state at 200 nm using time-resolved photoelectron spectroscopy and on-the-fly time-dependent density functional theory dynamics simulations. The
Guotao Li et al.
Journal of the American Chemical Society, 130(22), 6944-6945 (2008-05-10)
An efficient Au(I)-catalyzed synthesis of highly strained and functionalized bicyclo[3.2.0]heptanes is developed. Subsequent couplings with various nucleophiles offer additional structural features/complexity. These one-pot, three-component reactions are proposed to proceed via a key 1,3-dipolar cycloaddition between a Au carbenoid-containing carbonyl ylide
Natalia Chernyak et al.
Journal of the American Chemical Society, 134(30), 12466-12469 (2012-07-21)
Anilines and ethyl vinyl ether can be used as precursors for a process that is the synthetic equivalent of the α-arylation of acetaldehyde enolate. The reaction manifests a high level of functional group compatibility, allowing the ready preparation of a
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.