추천 제품
일반 설명
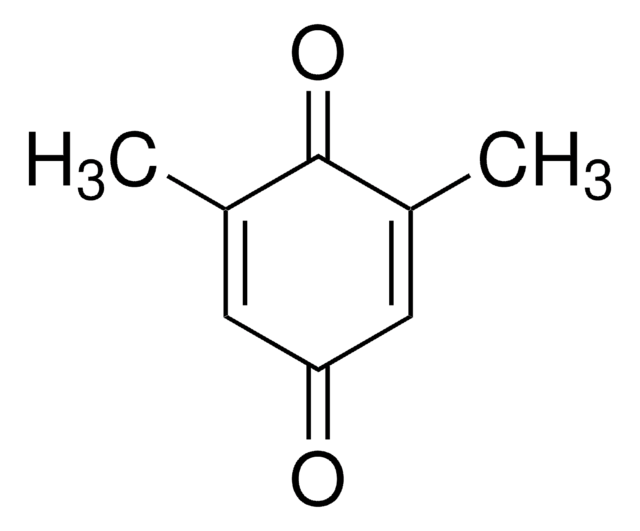
2,6-Dimethoxy-1,4-benzoquinone (DBQ, 2,6-DMBQ, DMOBQ) is a 1,4-benzoquinone derivative. It is a wood allergen, has been reported to cause various skin and mucosal symptoms on exposure to wood dusts. It is formed as a product due to the activity of bacterial Azospirillum lipoferum laccase on phenolic compounds of the syringic type. DBQ is one of the components isolated from the rhizome of Gynura japonica with a potential to show anti-platelet aggregation activity in vitro. It is an anticancer agent, whose kinetics of cyclic redox transformation induced by ascorbate (AscH-) has been studied using the Clark electrode and ESR techniques. Its electrochemical reduction in acetonitrile has been studied.
애플리케이션
2,6-Dimethoxy-1,4-benzoquinone may be used in the synthesis of 2-aryl-3,5-dimethoxy-1,4-benzoquinone derivatives.
Known haustorial inducing factor.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Phenolic derivatives related to lignin metabolism as substrates for Azospirillum laccase activity.
Faure D, et al.
Phytochemistry, 42(2), 357-359 (1996)
S P Wolff et al.
Experimental eye research, 45(6), 791-803 (1987-12-01)
The ability of 2,6-dimethoxyquinone (DMQ) to impair 86Rb uptake by bovine lens epithelial cells was found to be independent of exogenous ascorbate in contrast to the impairment induced by Fe/Cu or riboflavin plus light. The cytotoxicity was associated with an
Anomalous behavior in the two-step reduction of quinones in acetonitrile.
Lehmann MW and Evans DH.
Journal of Electroanalytical Chemistry, 500(1), 12-20 (2001)
Lelde Krumina et al.
Environmental science & technology, 51(16), 9053-9061 (2017-07-12)
Hydroquinones are important mediators of electron transfer reactions in soils with a capability to reduce Fe(III) minerals and molecular oxygen, and thereby generating Fenton chemistry reagents. This study focused on 2,6-dimethoxy hydroquinone (2,6-DMHQ), an analogue to a common fungal metabolite
Fang-Rong Chang et al.
Journal of natural products, 65(3), 255-258 (2002-03-23)
Three new eudesmanolide sesquiterpenes, neolitacumone A-C (1-3), and one new benzylisoquinoline alkaloid, neolitacumonine (5), along with 27 known compounds were isolated from the stem bark of Neolitsea acuminatissima. The structures of compounds 1-3 and 5 were established on the basis
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.