추천 제품
vapor pressure
12.46 psi ( 55 °C)
3.6 psi ( 20 °C)
Quality Level
형태
liquid
농도
1.0 M in THF
density
0.927 g/mL at 25 °C
저장 온도
2-8°C
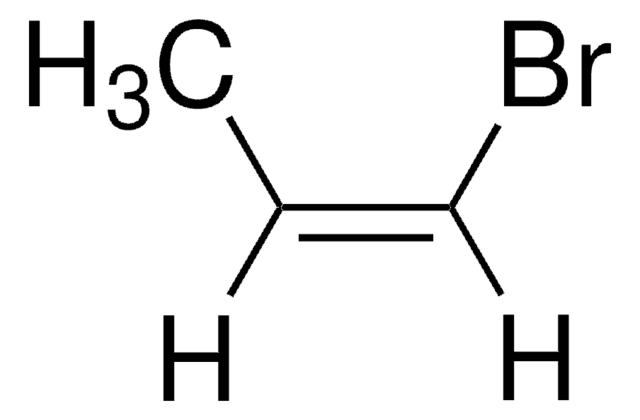
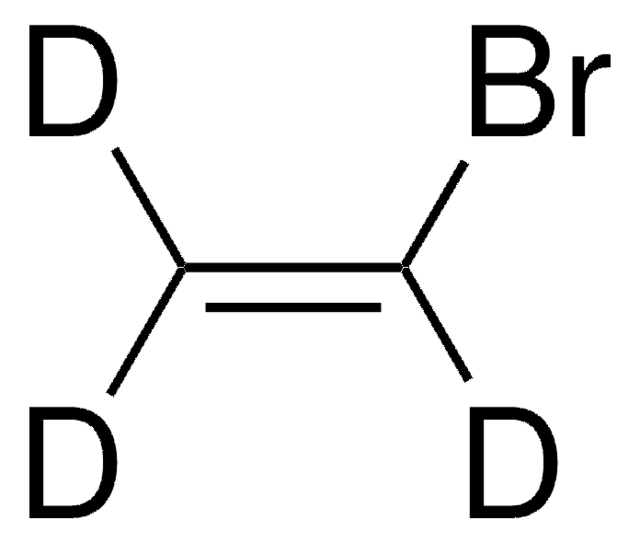
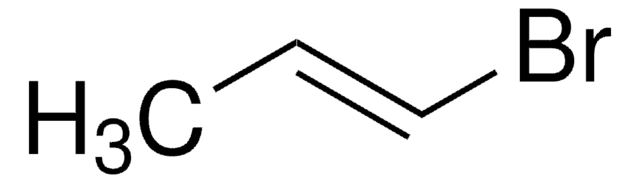
SMILES string
BrC=C
InChI
1S/C2H3Br/c1-2-3/h2H,1H2
InChI key
INLLPKCGLOXCIV-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Vinyl bromide solution belongs to haloalkenes andis highly reactive due to the presence of an unsaturated vinyl group. It can provide flame retardancy to polymers or materials when incorporated into their structure. It is also a versatile building block for polymerization, addition reactions, substitution reactions, and cross-coupling reactions like Suzuki-Miyaura and Negishi reactions. It can be used to introduce radiolabel into molecules for medical imaging.
애플리케이션
- Sequential Vinyl Radical Cyclization/Fixation of Carbon Dioxide through Electrochemical Reduction of Vinyl Bromide in the Presence of an Electron-Transfer Mediator: This study explores the electrochemical reduction of vinyl bromide with a focus on vinyl radical cyclization and carbon dioxide fixation (A Katayama, H Senboku, 2016).
- A Comparison of the Wavelength-Dependent Photochemical Reactions of Ozone with Vinyl Bromide and Fluoride in Argon Matrices: The study compares the photochemical reactions of vinyl bromide and fluoride with ozone, examining their behavior in argon matrices (BS Ault, 2021).
Vinyl bromide solution can be used as a precursor for stereoselective synthesis of chiral 2-vinyl-tetrahydronaphthalens via asymmetric reductive coupling. These chiral compounds are valuable building blocks for natural products, agrochemicals, and liquid crystals.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 1B - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
표적 기관
Central nervous system, Respiratory system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
1.4 °F - closed cup
Flash Point (°C)
-17 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Nickel/Copper Co-catalyzed Enantioselective Reductive Coupling of Oxabenzonorbornadienes with Vinyl Bromides
Yao Deng, et al.
advanced synthesis and catalysis, 365, 3265-3270 (2023)
Piotr Pawluć et al.
Organic letters, 11(15), 3390-3393 (2009-07-04)
A new, efficient protocol for the highly stereoselective one-pot synthesis of (E)-beta-aryl vinyl iodides and (E)-beta-aryl vinyl bromides from styrenes based on sequential ruthenium-catalyzed silylative coupling-N-halosuccinimide-mediated halodesilylation reactions is reported.
Cheon-Gyu Cho et al.
Organic letters, 7(16), 3569-3572 (2005-07-29)
An effective, readily scalable two-step synthesis of trisubstituted (E)-vinyl bromides involving bromination of alpha,beta-unsaturated lactones followed by hydrolytic fragmentation has been developed. Several trisubstituted (E)-vinyl bromides, including multigram quantities of (+)-(E)-4-bromo-2-methyl-3-pentenol, a synthetic intermediate required for the C(8)-C(11) moieties of
Changhui Sun et al.
Organic letters, 11(18), 4084-4087 (2009-08-20)
With the catalysis of CuI/trans-N,N'-dimethylcyclohexane-1,2-diamine, a number of carboxylic acids underwent efficient intramolecular O-vinylation with vinyl bromides leading to the synthesis of the corresponding five- and six-membered enol lactones. The same catalytic system also led to the efficient cycloisomerization of
Hsin-Lun Kao et al.
Organic letters, 13(19), 5204-5207 (2011-09-02)
The synthesis of vinyl sulfides through the coupling reaction of thiols with vinyl iodides, bromides, and chlorides is described. The thiols can couple with aryl iodides in the presence of only 0.5 mol % Cu(2)O without the need for an
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.