추천 제품
Quality Level
반응 적합성
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
파라미터
temperature sensitive
mp
60 °C (dec.) (lit.)
저장 온도
−20°C
SMILES string
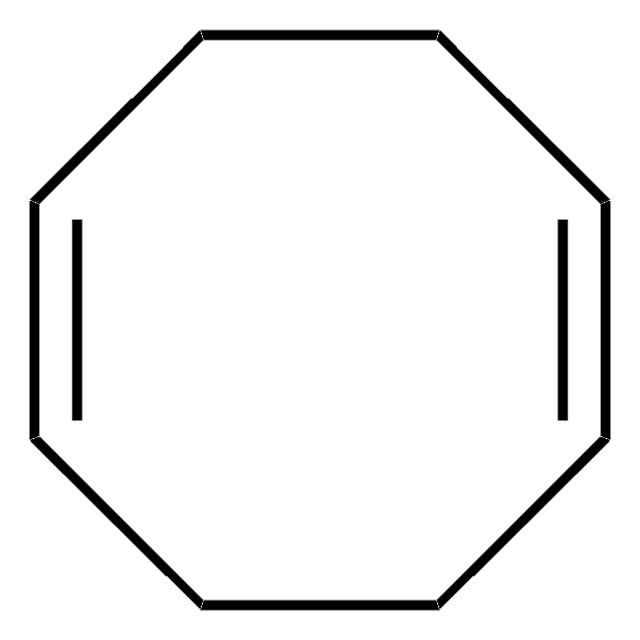
[Ni].C1CC=CCCC=C1.C2CC=CCCC=C2
InChI
1S/2C8H12.Ni/c2*1-2-4-6-8-7-5-3-1;/h2*1-2,7-8H,3-6H2;/b2*2-1-,8-7-;
InChI key
JRTIUDXYIUKIIE-KZUMESAESA-N
애플리케이션
- Oxidative addition reactions
Catalyst for:
- Asymmetric α-arylation and heteroarylation of ketones with chloroarenes
- Cross-coupling reactions
- Regioselective and stereoselective carboxylation/cyclization of allenyl aldehydes under a carbon dioxide atmosphere
- Methyl carboxylation of homopropargylic alcohols
- Stereoselective borylative ketone-diene coupling
- Cycloaddition of benzamides with internal alkynes
관련 제품
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Carc. 2 - Flam. Sol. 1 - Skin Sens. 1 - STOT RE 1
표적 기관
Lungs
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
문서
Effective Csp2- and Csp-hybridized coupling reactions established for catalysis, expanding to multi-step Csp3-coupling.
Nickel complexes catalyze various synthetic reactions like oxidative addition, C-H activation, and cross-coupling.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.