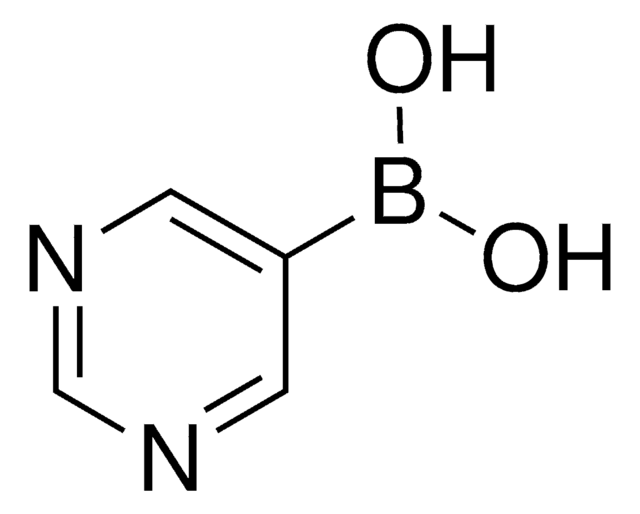
추천 제품
Quality Level
분석
≥95.0%
양식
solid
mp
>300 °C (lit.)
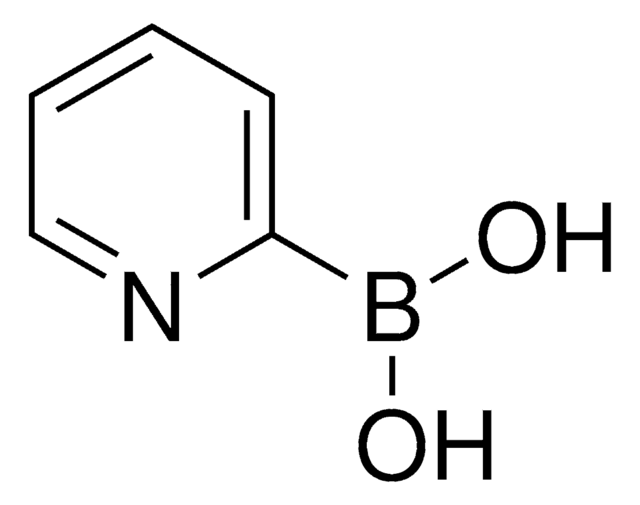
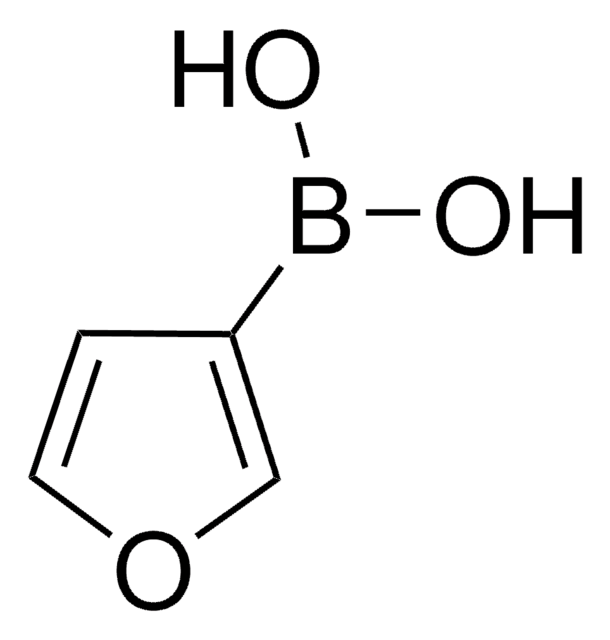
SMILES string
OB(O)c1cccnc1
InChI
1S/C5H6BNO2/c8-6(9)5-2-1-3-7-4-5/h1-4,8-9H
InChI key
ABMYEXAYWZJVOV-UHFFFAOYSA-N
애플리케이션
3-Pyridinylboronic acid can be used as a reagent for:
It can also be used to prepare:
- Phosphine-free Suzuki-Miyaura cross-coupling reactions.
- Regioselective Suzuki-Miyaura coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulation.
- N-arylation using copper acetylacetonate catalyst.
- Copper-mediated cyanation and regioselective cyanation of electron-rich benzenes.
It can also be used to prepare:
- New linear poly(phenylpyridyl) chains by Suzuki coupling.
- Oligopyridyl foldamers as mimics of a-helix twist.
- Many highly significant therapeutic enzymatic and kinase inhibitors and receptor antagonists.
- Pyridine substituted pyridinium N-(2′-azinyl)aminides by reacting with dibromo pyridinium aminides via Suzuki coupling reaction.
기타 정보
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Jana Sopkova-de Oliveira Santos et al.
Journal of chemical information and modeling, 52(2), 429-439 (2011-12-27)
Protein-protein interactions are central to many biological processes, from intracellular communication to cytoskeleton assembly, and therefore represent an important class of targets for new therapeutics. The most common secondary structure in natural proteins is an α-helix. Small molecules seem to
Regioselective Suzuki coupling on pyridinium N-(3,5-dibromoheteroar-2-yl)aminides
M. Jose R, et al.
Tetrahedron Letters, 47, 36, 6457-6460 (2006)
Synthesis of new linear poly(phenylpyridyl) chains
Perato, S.; et al.
Tetrahedron, 68, 1910-1917 (2012)
Justin I Montgomery et al.
Journal of medicinal chemistry, 55(4), 1662-1670 (2012-01-20)
The synthesis and biological activity of a new series of LpxC inhibitors represented by pyridone methylsulfone hydroxamate 2a is presented. Members of this series have improved solubility and free fraction when compared to compounds in the previously described biphenyl methylsulfone
Brian C Shook et al.
Journal of medicinal chemistry, 55(3), 1402-1417 (2012-01-14)
The design and characterization of two, dual adenosine A(2A)/A(1) receptor antagonists in several animal models of Parkinson's disease is described. Compound 1 was previously reported as a potential treatment for Parkinson's disease. Further characterization of 1 revealed that it was
문서
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.