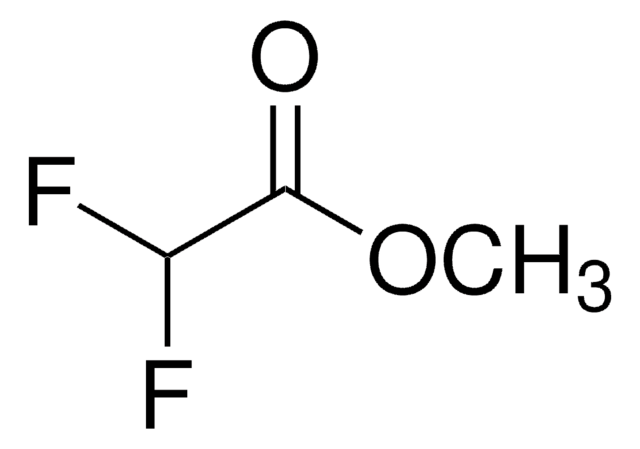
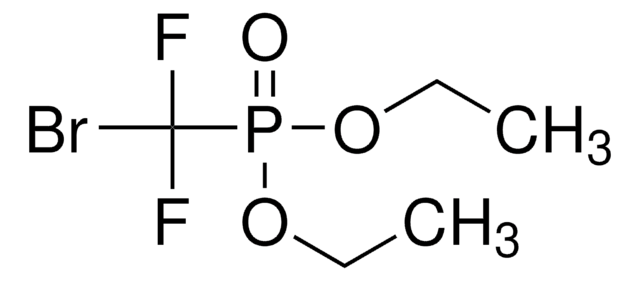
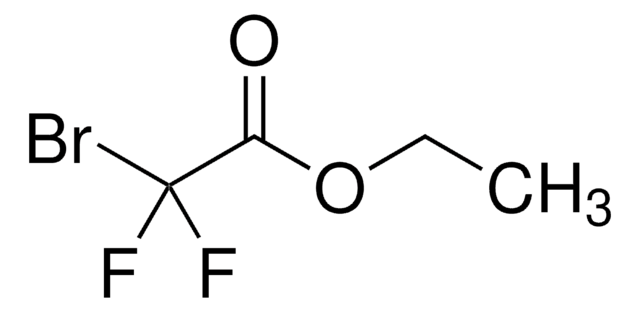
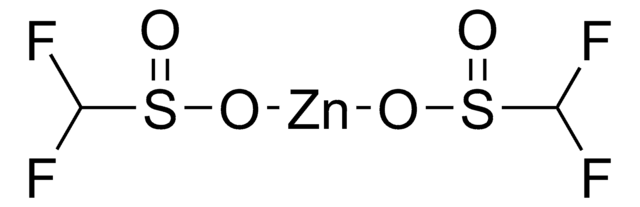
추천 제품
Quality Level
분석
97%
반응 적합성
reaction type: click chemistry
refractive index
n20/D 1.36 (lit.)
bp
153 °C (lit.)
density
1.723 g/mL at 25 °C (lit.)
작용기
carboxylic acid
fluoro
SMILES string
OC(=O)C(F)(F)S(F)(=O)=O
InChI
1S/C2HF3O4S/c3-2(4,1(6)7)10(5,8)9/h(H,6,7)
InChI key
VYDQUABHDFWIIX-UHFFFAOYSA-N
일반 설명
2,2-Difluoro-2-(fluorosulfonyl)acetic acid reagent is employed as a difluorocarbene source for difluoromethylation of phenolic hydroxyl groups.
애플리케이션
2,2-Difluoro-2-(fluorosulfonyl)acetic acid may be used in the following processes:
- Preparation of 1-difluoromethyl-2-oxo-1,2-dihydropyridine analogs by reacting with the corresponding 2-chloropyridines.
- Prepration of silyl fluorosulfonyldifluoroacetate as new highly efficient difluorocarbene reagent for cyclopropanation of alkenes.
- Regio- and stereoselective free radical fluoroalkylation of terminal alkenes and alkynes with iododifluoromethanesulfonamides.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Oral - Skin Corr. 1A
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
230.0 °F - closed cup
Flash Point (°C)
110 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Free radical fluoroalkylation of terminal alkenes and alkynes with iododifluoromethanesulfonamides.
Zhu JM, et al.
Science China: Chemistry, 54(1), 95-102 (2011)
Free radical fluoroalkylation of terminal alkenes and alkynes with iododifluoromethanesulfonamides
Zhu, J. M.; et al.
Science China: Chemistry, 54, 95-102 (2011)
Trimethylsilyl fluorosulfonyldifluoroacetate (TFDA): a new, highly efficient difluorocarbene reagent
Dolbier, William R.; et al.
Journal of Fluorine Chemistry, 125, 459-469 (2004)
Preparation and use of a new difluorocarbene reagent
Dolbier, W. R., Jr.; et al.
Organic Syntheses, 80, 172-176 (2003)
Makoto Ando et al.
Organic letters, 8(17), 3805-3808 (2006-08-11)
[reaction: see text] A novel one-pot synthesis of N-difluoromethyl-2-pyridones is described. N-(Pyridin-2-yl)acetamide derivatives were excellent precursors for the preparation of N-difluoromethyl-2-pyridone derivatives. Difluoromethylation of 2-acetaminopyridine derivatives was achieved with sodium chlorodifluoroacetate as a difluorocarbene source in the presence of a
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
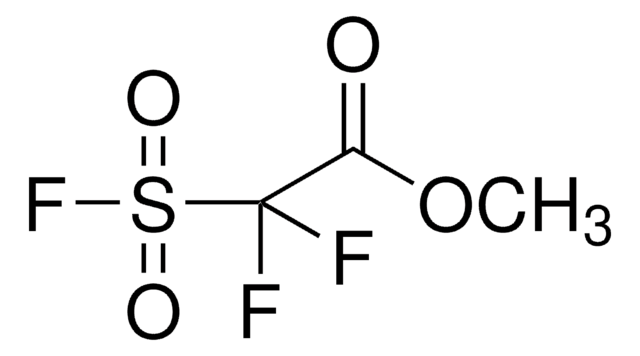
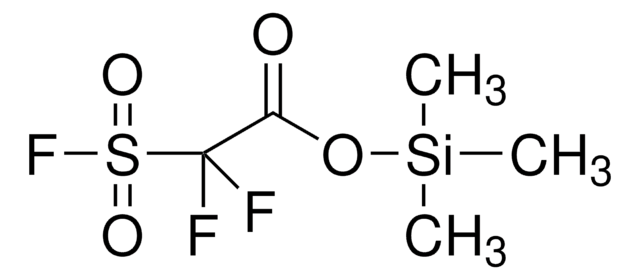


![4-(Acetylamino)phenyl]imidodisulfuryl difluoride ≥98%](/deepweb/assets/sigmaaldrich/product/structures/101/806/3f40354f-e903-4ea0-9654-10872377816c/640/3f40354f-e903-4ea0-9654-10872377816c.png)

![Zinc bis[bis(trimethylsilyl)amide] 97%](/deepweb/assets/sigmaaldrich/product/structures/294/819/cd22dd81-f7c8-4f0c-944e-1b74c1ad5e6d/640/cd22dd81-f7c8-4f0c-944e-1b74c1ad5e6d.png)