767840
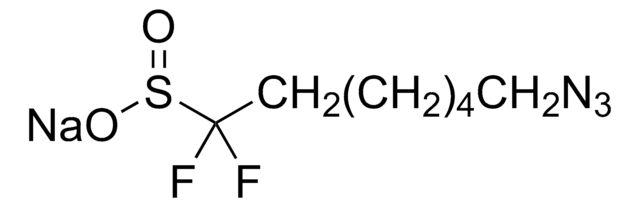
Zinc difluoromethanesulfinate
95%
동의어(들):
Bis(((difluoromethyl)sulfinyl)oxy)zinc, 1,1-difluoro-methanesulfinic acid zinc salt (2:1), Baran difluoromethylation reagent, DFMS
About This Item
추천 제품
Quality Level
분석
95%
양식
solid
반응 적합성
reaction type: C-C Bond Formation
reaction type: Fluorinations
reagent type: catalyst
reaction type: C-H Activation
reagent type: diversification reagent
작용기
fluoro
sulfinic acid
저장 온도
2-8°C
SMILES string
FC(F)S(=O)O[Zn]OS(=O)C(F)F
InChI
1S/2CH2F2O2S.Zn/c2*2-1(3)6(4)5;/h2*1H,(H,4,5);/q;;+2/p-2
InChI key
UGEYAPVLXKEKMP-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
Practical and Innate Carbon-Hydrogen Functionalization of Heterocycles
DFMS is a new reagent for direct difluoromethylation of organic substrates via a radical process. This mild, operationally simple, chemoselective, and scalable difluoromethylation method is compatible with a range of nitrogen-containing heteroarene substrates of varying complexity as well as select classes of conjugated p−systems and thiols.†
A New Reagent for Direct Difluoromethylation
Learn More at the Professor and Product Portal of Professor Phil S. Baran.
결합
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
문서
Rapidly diversify (hetero)aromatic scaffolds for chemical industry needs amid resource and time constraints, ensuring efficiency.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.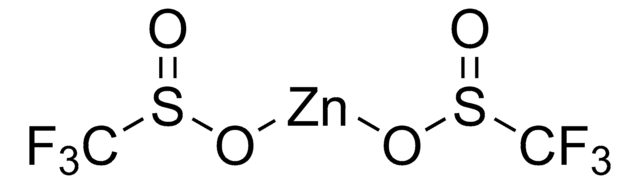

![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)