483869
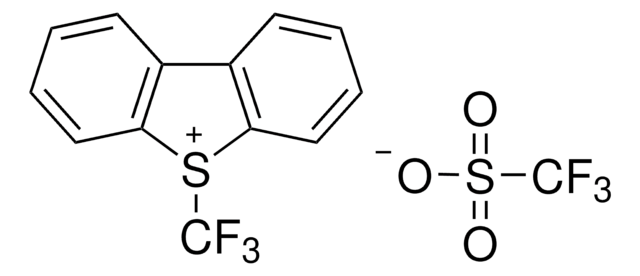
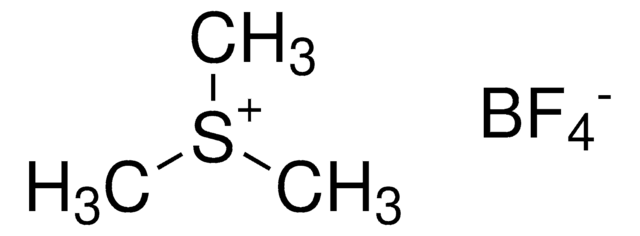
5-(Trifluoromethyl)dibenzothiophenium tetrafluoroborate
97%
동의어(들):
S-(Trifluoromethyl)dibenzothiophenium tetrafluoroborate, Umemoto reagent
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C13H8BF7S
CAS Number:
Molecular Weight:
340.07
MDL number:
UNSPSC 코드:
12352101
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
mp
162-164 °C (lit.)
작용기
fluoro
SMILES string
F[B-](F)(F)F.FC(F)(F)[S+]1c2ccccc2-c3ccccc13
InChI
1S/C13H8F3S.BF4/c14-13(15,16)17-11-7-3-1-5-9(11)10-6-2-4-8-12(10)17;2-1(3,4)5/h1-8H;/q+1;-1
InChI key
VTVISWLINKWMQZ-UHFFFAOYSA-N
애플리케이션
- Pd(II)-catalyzed trifluoromethylation
- Copper-catalyzed trifluoromethylation of aryl boronic acids using a Collidine as a trifluoromethylating reagent
- Pd-catalyzed electrophilic ortho-trifluoromethylation of arenes
Used in the stereoselective preparation of
- Trifluoromethyl-substituted alkenes via copper-catalyzed trifluoromethylation of terminal alkenes
- Trifluoromethyl-bearing quaternary carbon centers by Pd-catalyzed intramolecular decarboxylative allylation of α-trifluoromethyl β-keto esters
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Jun Xu et al.
Chemical communications (Cambridge, England), 47(14), 4300-4302 (2011-03-08)
A copper-catalyzed process for trifluoromethylation of aryl, heteroaryl, and vinyl boronic acids has been developed. The reaction is conducted under mild conditions and shows tolerance to moisture and a variety of functional groups.
Construction of Trifluoromethyl-Bearing Quaternary Carbon Centers by Intramolecular Decarboxylative Allylation of α-Trifluoromethyl β-Keto Esters
Shibata, N.; et al.
Advanced Synthesis & Catalysis, 353, 2037-2041 (2011)
Jun Xu et al.
Journal of the American Chemical Society, 133(39), 15300-15303 (2011-09-15)
An unprecedented type of reaction for Cu-catalyzed trifluoromethylation of terminal alkenes is reported. This reaction represents a rare instance of catalytic trifluoromethylation through C(sp(3))-H activation. It also provides a mechanistically unique example of Cu-catalyzed allylic C-H activation/functionalization. Both experimental and
Xing-Guo Zhang et al.
Journal of the American Chemical Society, 134(29), 11948-11951 (2012-07-12)
A Pd(II)-catalyzed trifluoromethylation of ortho C-H bonds with an array of N-arylbenzamides derived from benzoic acids is reported. N-Methylformamide has been identified as a crucial promoter of C-CF(3) bond formation from the Pd center. X-ray characterization of the C-H insertion
Xisheng Wang et al.
Journal of the American Chemical Society, 132(11), 3648-3649 (2010-02-27)
A Pd(II)-catalyzed C-H activation/trifluoromethylation of arenes with an electrophilic trifluoromethylation reagent using diverse heterocycle directing groups is reported. The presence of trifluoroacetic acid is crucial for this catalytic reaction.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.