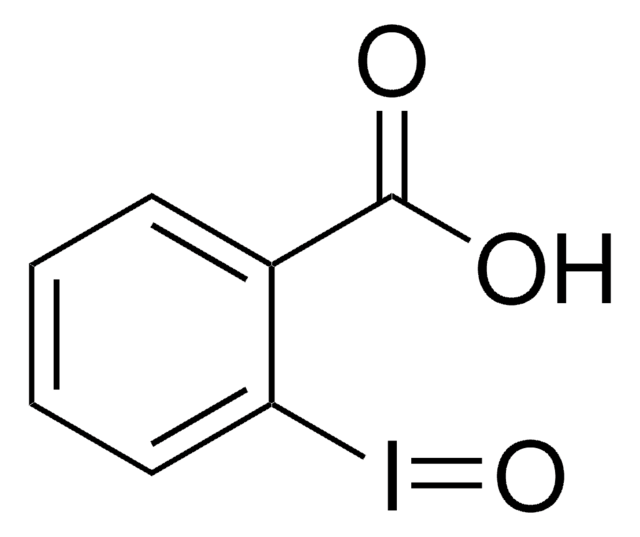
696641
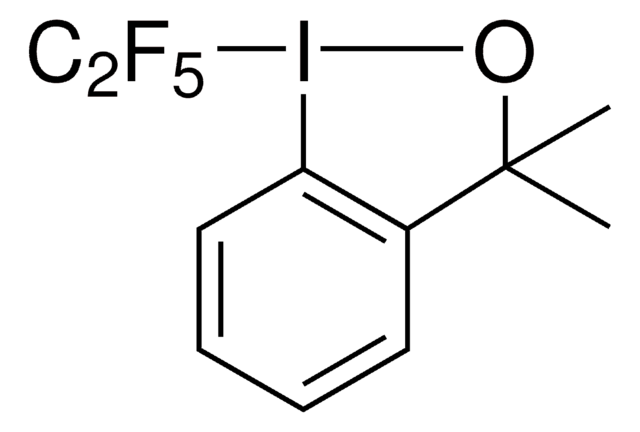
3,3-Dimethyl-1-(trifluoromethyl)-1,2-benziodoxole
95%
동의어(들):
1,3-Dihydro-3,3-dimethyl-1-(trifluoromethyl)-1,2-benziodoxole, Togni’s Reagent
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C10H10F3IO
CAS Number:
Molecular Weight:
330.09
MDL number:
UNSPSC 코드:
12352101
PubChem Substance ID:
NACRES:
NA.22
추천 제품
애플리케이션
Easily accessible hypervalent iodine compound acting as an electrophilic CF3-transfer reagent for direct, mild, and efficient trifluoromethylation.
Trifluoromethylation of a variety of compounds including:
Trifluoromethylation of a variety of compounds including:
- Secondary and primary aryl- and alkylphospines
- Phenols
- Peptides containing cysteine residudes by SPPS and electrophilic S-trifluoromethylation
- Arenes and N-heterocycles
- Electrophilic N-trifluoromethylation of Arozoles
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Electrophilic trifluoromethylation of arenes and N-heteroarenes using hypervalent iodine reagents
Wiehn, M. S.; Vinogradova, E. V.; Togni, A.
Journal of Fluorine Chemistry, 131, 951-957 (2010)
Patrick Eisenberger et al.
Chemical communications (Cambridge, England), 13(13), 1575-1577 (2008-03-21)
A direct, mild and efficient trifluoromethylation of primary and secondary phosphines is achieved with easily accessible, cheap hypervalent iodine compounds acting as electrophilic CF(3)-transfer reagents.
Katrin Niedermann et al.
Angewandte Chemie (International ed. in English), 51(26), 6511-6515 (2012-05-23)
Effective CF(3) transfer: Various electron-rich nitrogen heterocycles (pyrazoles, triazoles, and tetrazoles) can be directly N-trifluoromethylated by a hypervalent iodine reagent in an efficient manner. The optimized procedure, which includes an in situ silylation of the substrate followed by an acid-catalyzed
Electrophilic S-trifluoromethylation of cysteine side chains in a- and ?-peptides: isolation of trifluoromethylated Sandostatin (octreotide) derivatives
Capone, S.; et al.
Helvetica Chimica Acta, 91, 2035-2056 (2008)
Reactivity of a 10-I-3 Hypervalent Iodine Trifluoromethylation Reagent With Phenols
Stanek, K.; Koller, R.; Togni, A.
The Journal of Organic Chemistry, 19, 7678-7685 (2008)
문서
Fluoroalkylation toolbox expands with various reagents for late-stage fluoroalkylation in organic synthesis and medicinal chemistry.
관련 콘텐츠
Research in the Togni group focuses on the development of new ligands and reagents. These two general directions thus impact the ability to construct molecules in more efficient or unprecedented ways.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.







![1-[(Triisopropylsilyl)ethynyl]-1,2-benziodoxol-3(1H)-one ≥98.0% (AT)](/deepweb/assets/sigmaaldrich/product/structures/306/080/5d7d692f-a7c1-44ce-a2f9-ee6cffe8f42d/640/5d7d692f-a7c1-44ce-a2f9-ee6cffe8f42d.png)