568600
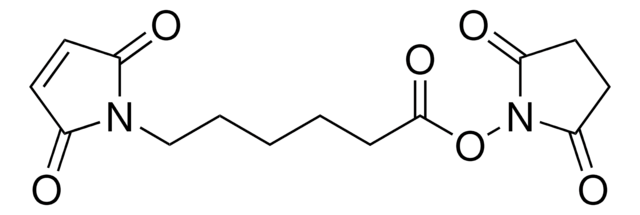
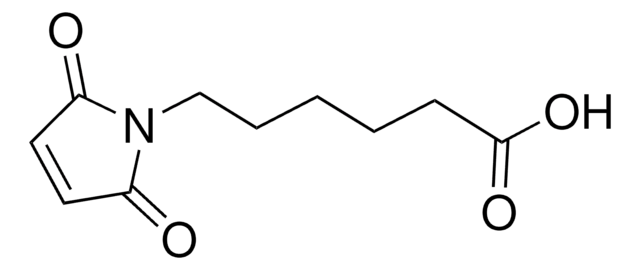
6-Maleimidohexanoic acid N-hydroxysuccinimide ester
98%
동의어(들):
6-Maleimidocaproic acid N-succinimidyl ester, N-(ε-Maleimidocaproyloxy)succinimide, N-Succinimidyl 6-maleimidocaproate, EMCS
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C14H16N2O6
CAS Number:
Molecular Weight:
308.29
Beilstein:
1499815
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
반응 적합성
reagent type: cross-linking reagent
mp
70-73 °C (lit.)
작용기
NHS ester
imide
maleimide
저장 온도
−20°C
SMILES string
O=C(ON(C(CC1)=O)C1=O)CCCCCN2C(C=CC2=O)=O
InChI
1S/C14H16N2O6/c17-10-5-6-11(18)15(10)9-3-1-2-4-14(21)22-16-12(19)7-8-13(16)20/h5-6H,1-4,7-9H2
InChI key
VLARLSIGSPVYHX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
6-Maleimidohexanoic acid N-hydroxysuccinimide ester can be used in:
- Synthesis of maleimide-activated carbohydrates for site-specific glycosylation of cysteine-containing peptides and proteins via maleimide-thiol ligation reaction.
- Synthesis of a glucuronide prodrug of doxorubicin bearing a maleimide side chain as an antitumor agent.
- Cross-linking oligonucleotides with the amino groups on the substrate to fabricate DNA microarrays.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Synthesis and antitumor efficacy of a β-glucuronidase-responsive albumin-binding prodrug of doxorubicin.
Legigan T, et al.
Journal of Medicinal Chemistry, 55(9), 4516-4520 (2012)
Microarray fabrication with covalent attachment of DNA using bubble jet technology.
Okamoto T, et al.
Nature Biotechnology, 18(4), 438-438 (2000)
Synthesis of maleimide-activated carbohydrates as chemoselective tags for site-specific glycosylation of peptides and proteins.
Ni J, et al.
Bioconjugate Chemistry, 14(1), 232-238 (2003)
Y Nakano et al.
International archives of allergy and immunology, 120(3), 199-208 (1999-12-11)
We have previously reported that ovalbumin (OVA) coupled with liposome via glutaraldehyde (GA) induced OVA-specific- and IgE-selective unresponsiveness in mice. In this study, OVA-liposome conjugates were made using four different coupling protocols: via GA, N-(6-maleimidocaproyloxy) succinimide (EMCS), disuccinimidyl suberate (DSS)
N J Maeji et al.
Journal of immunological methods, 146(1), 83-90 (1992-01-21)
Recently, the multipin approach for simultaneous multiple peptide synthesis was applied to the analysis of T cell determinants by using a novel cleavage method (Maeji et al., 1990). A diketopiperazine forming linker allowed cleavage of peptides into aqueous buffer which
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.