638617
2-(Tri-n-butylstannyl)oxazole
동의어(들):
2-(Tributylstannanyl)-1,3-oxazole, 2-(Tributylstannanyl)oxazole, 2-(Tributylstannyl)-1,3-oxazole, 2-(Tributylstannyl)oxazole, Tributyl(oxazol-2-yl)stannane
About This Item
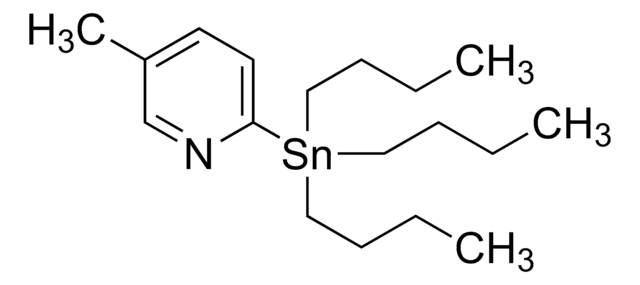
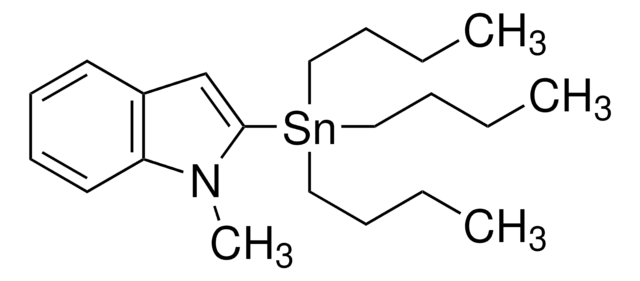
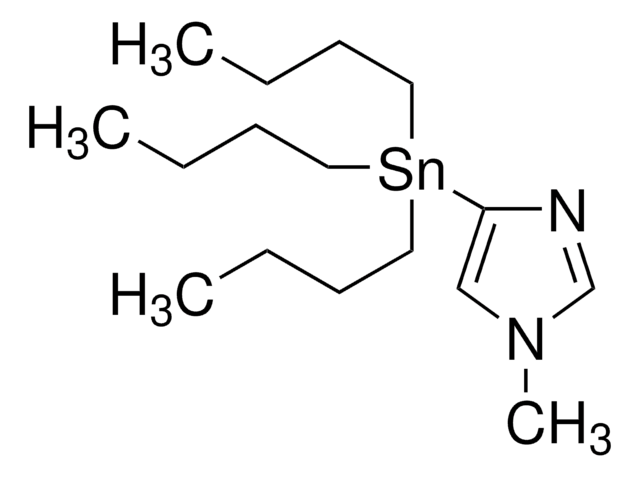
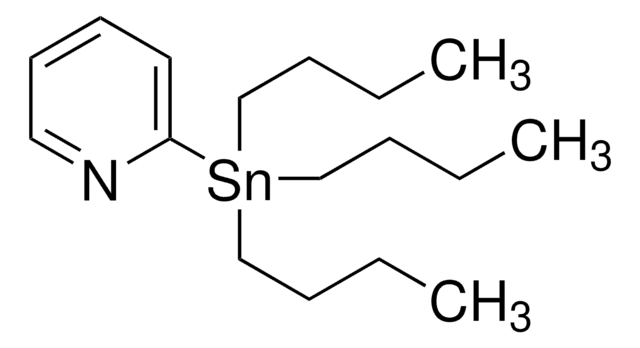
추천 제품
refractive index
n20/D 1.4930 (lit.)
Quality Level
bp
108-110 °C/0.2 mmHg (lit.)
density
1.170 g/mL at 25 °C
1.71 g/mL at 25 °C (lit.)
SMILES string
CCCC[Sn](CCCC)(CCCC)c1ncco1
InChI
1S/3C4H9.C3H2NO.Sn/c3*1-3-4-2;1-2-5-3-4-1;/h3*1,3-4H2,2H3;1-2H;
InChI key
YOWGRWHKDCHINP-UHFFFAOYSA-N
일반 설명
애플리케이션
- Heteroaromatic compounds via Stille-Migita cross-coupling reaction with (hetero)aryl halides using a palladium catalyst.
- Ethyl 2-[3-(1,3-oxazol-2-yl)-1H-indazol-1-yl]acetate by reacting with 3-iodoindazole in the presence of Pd(PPh3)4 as a catalyst.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)