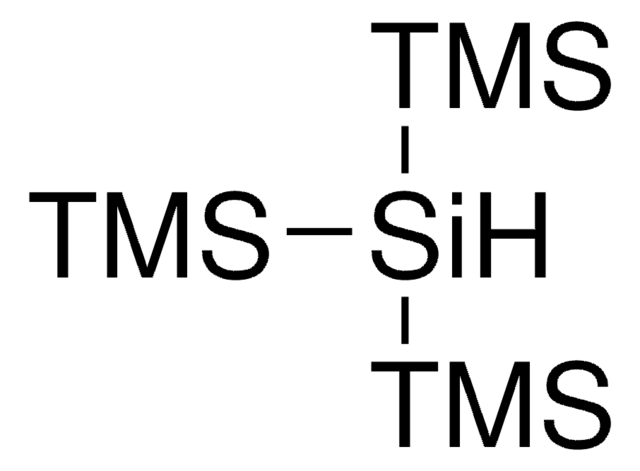736856
Tris(triethylsilyl)silane
동의어(들):
1,1,1,3,3,3-Hexaethyl-2-(triethylsilyl)trisilane, 3,4,5-Trisilaheptane, 3,3,5,5-tetraethyl-4-(triethylsilyl)
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C18H46Si4
CAS Number:
Molecular Weight:
374.90
MDL number:
UNSPSC 코드:
12352001
PubChem Substance ID:
NACRES:
NA.22
추천 제품
형태
liquid
Quality Level
반응 적합성
reagent type: reductant
refractive index
n20/D 1.526
density
0.887 g/mL at 25 °C
저장 온도
2-8°C
SMILES string
CC[Si](CC)(CC)[SiH]([Si](CC)(CC)CC)[Si](CC)(CC)CC
InChI
1S/C18H46Si4/c1-10-20(11-2,12-3)19(21(13-4,14-5)15-6)22(16-7,17-8)18-9/h19H,10-18H2,1-9H3
InChI key
WNGZMQFMMHZKBG-UHFFFAOYSA-N
애플리케이션
Tris(triethylsilyl)silane can be incorporated as a directing group for various regio- and stereo-selective reactions. Hydrogen abstraction from tris(triethylsilyl)silane yields highly stable silyl radical.
Tris(triethylsilyl)silane can be used as a hydrogen atom donor reagent in chemical synthesis due to its weak Si-H bond. Hydrogen abstraction from tris(triethylsilyl)silane yields a highly stable silyl radical.
It can be used as a reagent:
It can be used as a reagent:
- In the radical coupling reaction to generate C-C bonds from alkyl-halogen compounds using iridium and nickel catalysts.
- To synthesize α-arylated product via cross-electrophile coupling reaction between α-chloro carbonyl and aryl bromide in the presence of nickel and iridium catalysts.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Tiffany Q Chen et al.
Angewandte Chemie (International ed. in English), 58(41), 14584-14588 (2019-08-15)
Here, we demonstrate that a metallaphotoredox-catalyzed cross-electrophile coupling mechanism provides a unified method for the α-arylation of diverse activated alkyl chlorides, including α-chloroketones, α-chloroesters, α-chloroamides, α-chlorocarboxylic acids, and benzylic chlorides. This strategy, which is effective for a wide variety of
Jonathan D Bell et al.
Chemical Society reviews, 50(17), 9540-9685 (2021-07-27)
Photoredox chemistry with organic or transition metal agents has been reviewed in earlier years, but such is the pace of progress that we will overlap very little with earlier comprehensive reviews. This review first presents an overview of the area
Controlling stereochemistry in polyketide synthesis: 1, 3-vs. 1, 2-asymmetric induction in methyl ketone aldol additions to ?-super siloxy aldehydes.
Brady PB, et al.
Chemical Science, 4(8), 3223-3231 (2013)
Highly Stable Silyl Radicals (Et n Me3-n Si) 3Si?(n= 1? 3).
Kyushin S, et al.
Organometallics, 16(25), 5386-5388 (1997)
Generation of Organolithium Compounds bearing Super Silyl Ester and their Application to Matteson Rearrangement.
Oda S and Yamamoto H
Angewandte Chemie (International Edition in English), 125(31), 8323-8326 (2013)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.