추천 제품
Quality Level
분석
95%
형태
powder or crystals
반응 적합성
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
작용기
phosphine
관련 카테고리
애플리케이션
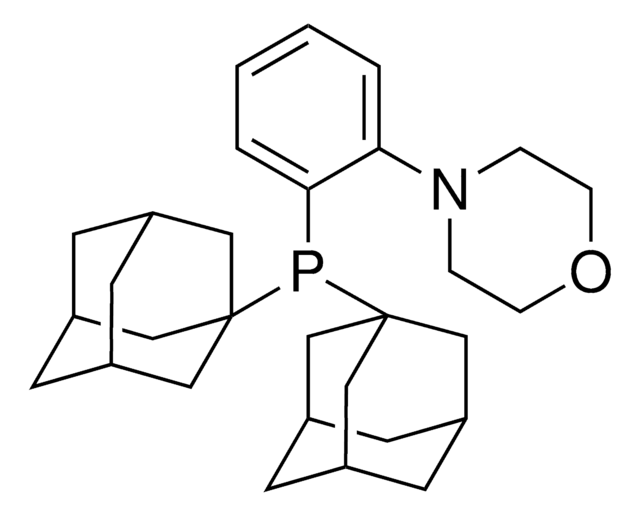
PAd-DalPhos is a versatile air stable pre-catalyst for C(sp2)-N coupling. It catalyzes N-arylation of amides with (hetero)aryl (pseudo)halide. It can also be used in the synthesis of unsymmetrical 1,3-di(hetero)aryl-1H-indazoles from hydrazine, o-chloro (hetero)benzophenones and (hetero)aryl bromides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Ni and Cu-catalyzed one pot synthesis of unsymmetrical 1, 3-di (hetero) aryl-1H-indazoles from hydrazine, o-chloro (hetero) benzophenones, and (hetero) aryl bromides.
Wiethan C, et al.
Organic & Biomolecular Chemistry, 15(23), 5062-5069 (2017)
Nickel?Catalyzed N?Arylation of Primary Amides and Lactams with Activated (Hetero) aryl Electrophiles.
Lavoie C M, et al.
Chemistry?A European Journal , 22(52), 18752-18755 (2016)
Christopher M Lavoie et al.
Nature communications, 7, 11073-11073 (2016-03-24)
Palladium-catalysed C(sp(2))-N cross-coupling (that is, Buchwald-Hartwig amination) is employed widely in synthetic chemistry, including in the pharmaceutical industry, for the synthesis of (hetero)aniline derivatives. However, the cost and relative scarcity of palladium provides motivation for the development of alternative, more
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.



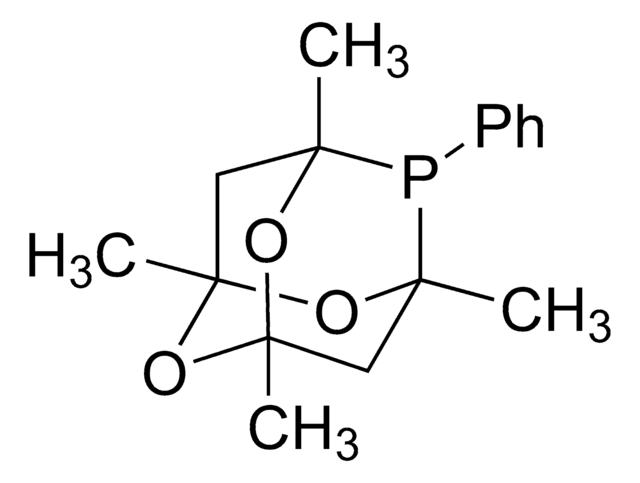
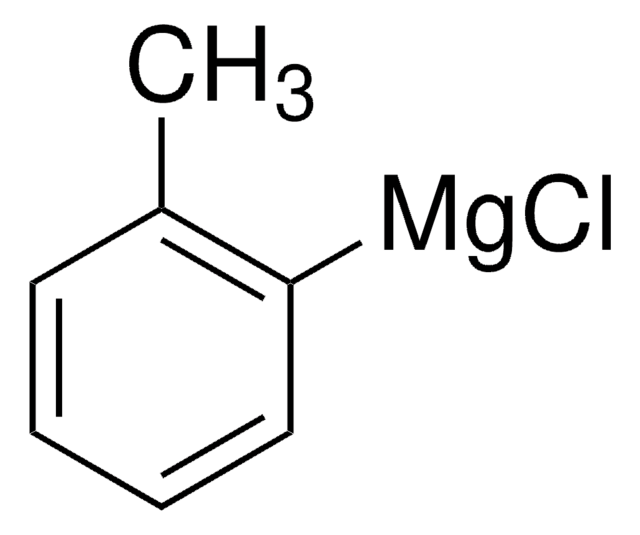

![5-[Di(1-adamantyl)phosphino]-1′,3′,5′-triphenyl-1′H-[1,4′]bipyrazole 97%](/deepweb/assets/sigmaaldrich/product/structures/334/020/2da77724-fc86-4d88-bc93-0b43ae777daa/640/2da77724-fc86-4d88-bc93-0b43ae777daa.png)
![[(1,3,5,7-Tetramethyl-6-phenyl-2,4,6-trioxa-6-phosphaadamantane)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate](/deepweb/assets/sigmaaldrich/product/structures/324/001/3ffb4bd2-9c6b-451c-80ee-a217f03ca932/640/3ffb4bd2-9c6b-451c-80ee-a217f03ca932.png)
