900873
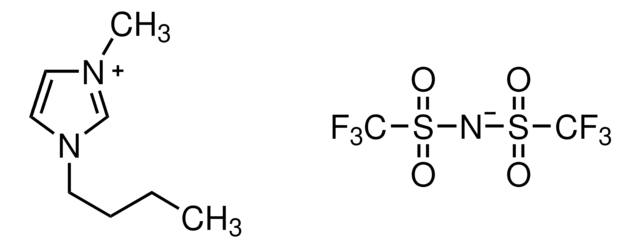
1-Butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide
>99%, <500 ppm H2O
동의어(들):
N,N-Butylmethylpyrrolidinium bis(trifluoromethanesulfonyl)amide, N,N-Butylmethylpyrrolidinium bis(trifluoromethanesulfonyl)imide, N,N-Butylmethylpyrrolidinium trifluoromethanesulfonimide, PYR14-TFSI
About This Item
추천 제품
Quality Level
분석
>99%
양식
liquid
불순물
<500 ppm H2O
mp
-18 °C
density
1.378 g/cm3
응용 분야
battery manufacturing
SMILES string
CCCC[N+]1(C)CCCC1.FC(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)F
InChI
1S/C9H20N.C2F6NO4S2/c1-3-4-7-10(2)8-5-6-9-10;3-1(4,5)14(10,11)9-15(12,13)2(6,7)8/h3-9H2,1-2H3;/q+1;-1
InChI key
HSLXOARVFIWOQF-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
관련 제품
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
>230.0 °F - Not applicable
Flash Point (°C)
> 110 °C - Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
문서
Solid-state Li batteries: Review of solid electrolytes, ion conduction, structures, and electrochemical processes.
Ionic liquid electrolytes explored for rechargeable batteries' advancement; future IL development discussed.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.