추천 제품
양식
powder or crystals
반응 적합성
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
mp
>300 °C
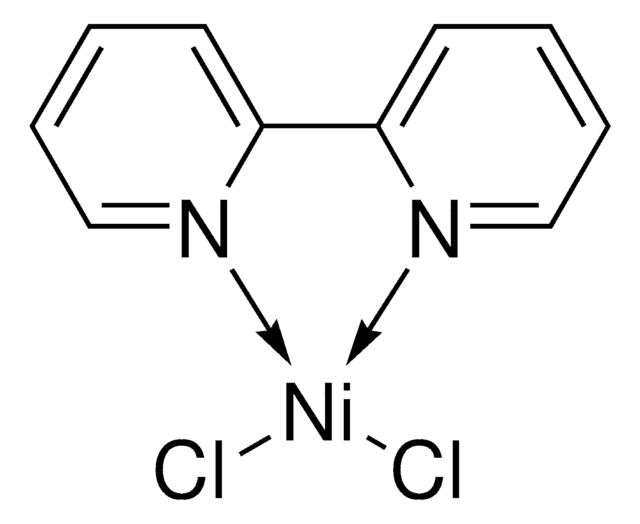
SMILES string
CC(C1=CC(C2=CC(C(C)(C)C)=CC=N2)=NC=C1)(C)C.Cl[Ni]Cl
InChI key
PCWIKFRTCXESOT-UHFFFAOYSA-L
애플리케이션
[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride can be used as a catalyst in:
- Decarboxylative arylation of oxo acids.
- Acylation of ethers.
- Cross-coupling of aryl bromides with alcohols.
관련 제품
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Wacharee Harnying et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(17), 4765-4773 (2011-03-23)
The roles of nickel and chromium catalysts in the coupling reaction of vinyl halides and aldehydes, the so-called Nozaki-Hiyama-Kishi (NHK) reaction, have been studied by UV/Vis spectroscopy, electrochemical, and spectroelectrochemical methods. Electrochemical studies revealed that nickel plays the central role
Lingling Chu et al.
Angewandte Chemie (International ed. in English), 54(27), 7929-7933 (2015-05-28)
The direct decarboxylative arylation of α-oxo acids has been achieved by synergistic visible-light-mediated photoredox and nickel catalysis. This method offers rapid entry to aryl and alkyl ketone architectures from simple α-oxo acid precursors via an acyl radical intermediate. Significant substrate
Aryl Ketones as Single-Electron-Transfer Photoredox Catalysts in Nickel-Catalyzed the Homocoupling of Aryl Halides
Masuda Y, et al.
European Journal of Organic Chemistry, 5822-5825 (2016)
Photocatalytic α-Acylation of Ethers
Sun Z, et al.
Organic Letters, 19, 3727-3730 (2017)
Merging photoredox and nickel catalysis: The direct synthesis of ketones by the decarboxylative arylation of α-oxo acids
Chu L, et al.
Angewandte Chemie (International ed. in English), 127, 8040-8044 (2015)
문서
Nickel complexes catalyze various synthetic reactions like oxidative addition, C-H activation, and cross-coupling.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)



![[Ni(dtbbpy)(H2O)4]Cl2](/deepweb/assets/sigmaaldrich/product/structures/777/629/15c13300-e874-4abd-8bd4-8b2bb4864570/640/15c13300-e874-4abd-8bd4-8b2bb4864570.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![[Ir(dF(Me)ppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)

![Bis[(2-dimethylamino)phenyl]amine nickel(II) chloride ≥97% (AT)](/deepweb/assets/sigmaaldrich/product/structures/143/670/3d0cc911-c810-4324-914e-85c5c11b7dac/640/3d0cc911-c810-4324-914e-85c5c11b7dac.png)
![[(TMEDA)Ni(o-tolyl)Cl] 95%](/deepweb/assets/sigmaaldrich/product/structures/236/439/768c916e-994f-47e3-a980-3ca0471317d7/640/768c916e-994f-47e3-a980-3ca0471317d7.png)
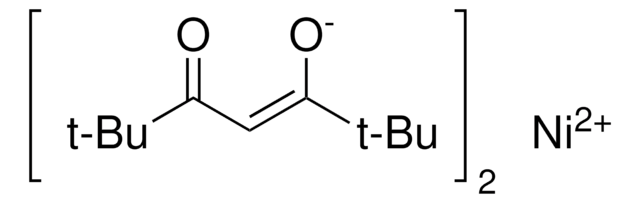
![[1,3-Bis(diphenylphosphino)propane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)