추천 제품
Grade
certified reference material
Quality Level
양식
liquid
특징
Snap-N-Spike®/Snap-N-Shoot®
포장
ampule of 1 mL
제조업체/상표
Cerilliant®
농도
1.0 mg/mL in acetonitrile
기술
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
응용 분야
clinical testing
형식
single component solution
저장 온도
−70°C
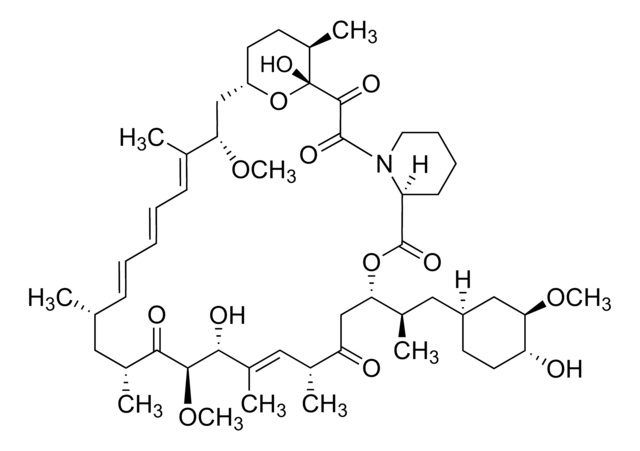
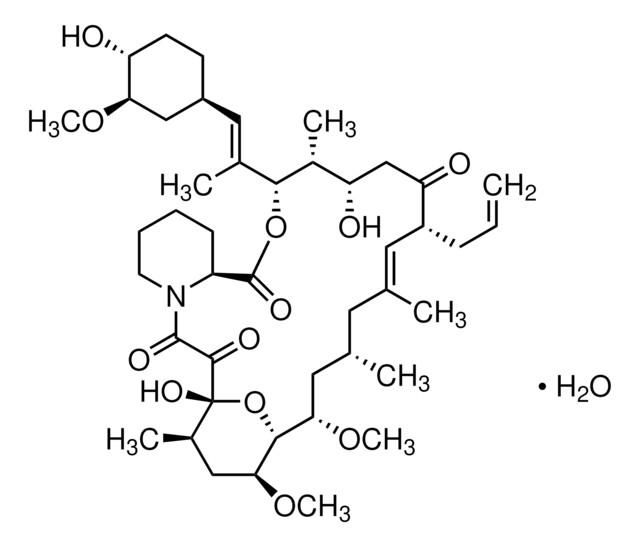
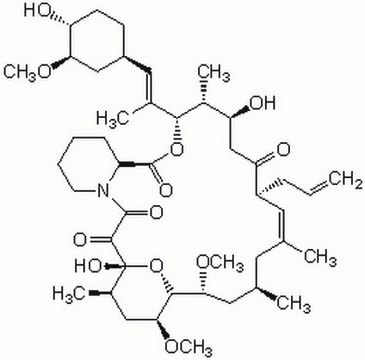
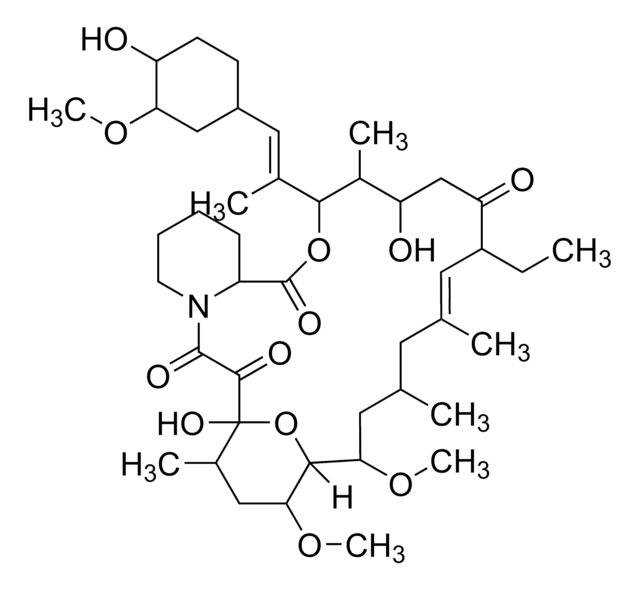
SMILES string
CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]2OC(=O)[C@@H]3CCCCN3C(=O)C(=O)[C@]4(O)O[C@H]([C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)OC)[C@H](C[C@H]4C)OC
InChI
1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1
InChI key
QJJXYPPXXYFBGM-LFZNUXCKSA-N
유전자 정보
human ... FKBP1A(2280)
일반 설명
애플리케이션
- Forensic toxicology and clinical implications: An analysis of AB-PINACA′s metabolites in solid tissues from an abuser, compared with in vitro experiments, highlights the compound′s potent effects and underscores its relevance in toxicological and forensic investigations (Minakata et al., 2021).
법적 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
35.6 °F - closed cup
Flash Point (°C)
2 °C - closed cup
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.