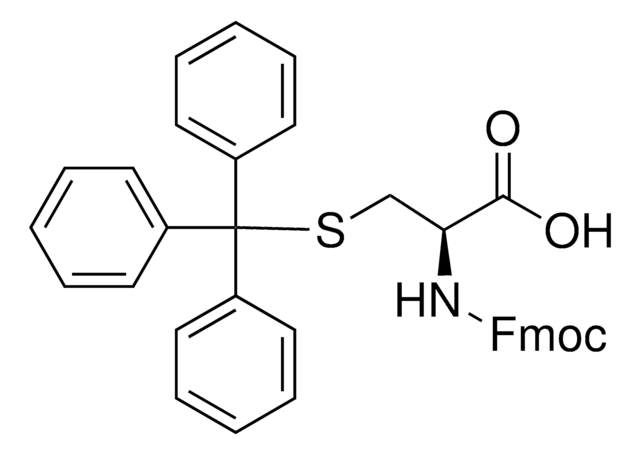
추천 제품
product name
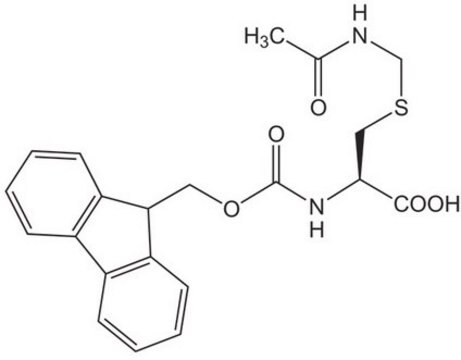
Fmoc-Cys(STmp)-OH, Novabiochem®
Quality Level
제품 라인
Novabiochem®
형태
powder
반응 적합성
reaction type: Fmoc solid-phase peptide synthesis
제조업체/상표
Novabiochem®
응용 분야
peptide synthesis
작용기
thiol
저장 온도
15-25°C
일반 설명
Fmoc-Cys(STmp)-OH is a novel new tool for the regioselective synthesis of multiple disulfide bridged peptides by Fmoc SPPS. The STmp group is stable to piperidine but is extremely easy to remove by mild thiolysis. Albericio has reported removing four STmp groups on the solid phase with only three 5 minute treatments of 0.1 M N-methylmorpholine (NMM) in DMF containing 5% DTT.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Fmoc SPPS of Cysteine-Containing Peptides
Literature references:
[1] T. M. Postma, et al. (2012) Org. Lett., 14, 5468.
[2] T. M. Postma & F. Albericio (2013) Org. Lett., 15, 616.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Fmoc SPPS of Cysteine-Containing Peptides
Literature references:
[1] T. M. Postma, et al. (2012) Org. Lett., 14, 5468.
[2] T. M. Postma & F. Albericio (2013) Org. Lett., 15, 616.
애플리케이션
Applications of Fmoc-Cys(STmp)-OH:
- Synthesis of insulin analogs by regiospecific disulfide bond formation.
- A review on step-wise introduction of disulfide bonds.
- Synthesis of human insulin-like peptide 6.
분석 메모
Color (visual): white to slight yellow to beige
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.5 % (a/a)
Assay (HPLC, area%): ≥ 94.0 % (a/a)
Purity (TLC(011A)): ≥ 98 %
Solubility (1 mmole in 2 ml DMF): clearly soluble
Ethyl acetate (HS-GC): ≤ 0.5 %
Acetate (IC): ≤ 0.05 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.5 % (a/a)
Assay (HPLC, area%): ≥ 94.0 % (a/a)
Purity (TLC(011A)): ≥ 98 %
Solubility (1 mmole in 2 ml DMF): clearly soluble
Ethyl acetate (HS-GC): ≤ 0.5 %
Acetate (IC): ≤ 0.05 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
법적 정보
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
N-Chlorosuccinimide, an efficient reagent for on-resin disulfide formation in solid-phase peptide synthesis
T. M. Postma & F. Albericio
Organic Letters, 15, 616-616 (2013)
Chemical Synthesis of Human Insulin-Like Peptide-6
Chemistry?A European Journal , 22, 9777-9777 (2016)
Trimethoxyphenylthio as a highly labile replacement for tert-butylthio cysteine protection in Fmoc solid phase synthesis
T. M. Postma, et al.,
Organic Letters, 14, 5468-5468 (2012)
Synthesis of Four-Disulfide Insulin Analogs via Sequential Disulfide Bond Formation
Fangzhou Wu, et al.
The Journal of Organic Chemistry, 82, 3506-3506 (2017)
Stepwise Construction of Disulfides in Peptides
H Rongjun, et al.,
Chembiochem, 21, 1101-1101 (2020)
문서
Novabiochem® offers orthogonally protected amino acids for peptide synthesis, including cyclic and branched peptides.
프로토콜
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.