추천 제품
생물학적 소스
bovine lung
Quality Level
양식
lyophilized
포장
pkg of 10 mg (10236624001)
pkg of 100 mg (11583794001)
pkg of 50 mg (10981532001)
제조업체/상표
Roche
기술
electrophoresis: suitable
tissue culture: suitable
pH 범위
3-10
solubility
water: soluble 10 mg/mL
흡수
0.84 at 280 nm
배송 상태
wet ice
저장 온도
2-8°C
관련 카테고리
일반 설명
Trypsin inhibitor, pancreas type from bovine lung. It is known as Pancreatic trypsin inhibitor (BPTI). Aprotinin, also known as pancreatic trypsin inhibitor and trypsin-kallikrein inhibitor, is found to be expressed in lungs, spleen, liver, and pancreas. It is also found to be present in the free form in calf serum.
특이성
Aprotinin inhibits serine proteases. It inhibits kallikrein, the protease that releases hypotensive peptides such as kallidin and bradykinin (human plasma kallikrein: Ki = 3 ×10-8 M at pH 8.0, porcine pancreas kallikrein: Ki = 1 × 10-9 M at pH 8.0), trypsin (Ki = 2.8 × 10-11 M at pH 7.8, Ki = 2.6 × 10-9 M at pH 4.0, non-competetive), trypsinogen, chymotrypsin (Ki = 9 × 10-9 M at pH 8.0), bacterial fibrinolysin, and plasmin (Ki = 1 nM at pH 7.3).
Cathepsin G, acrosin, human leukocyte elastase, and human urokinase are weakly inhibited. Factor Xa, thrombin, subtilisin, papain, pepsin, angiotensin-converting enzyme (ACE), carboxypeptidase A and B, other metalloproteases, and thiolproteases are not inhibited.
Cathepsin G, acrosin, human leukocyte elastase, and human urokinase are weakly inhibited. Factor Xa, thrombin, subtilisin, papain, pepsin, angiotensin-converting enzyme (ACE), carboxypeptidase A and B, other metalloproteases, and thiolproteases are not inhibited.
애플리케이션
Aprotinin is used for the protection of proteins and enzymes during isolation/purification. The inhibition of protease activity increases the lifetime of cells in cell and tissue culture studies.
- Further applications: Purification of urokinase, trypsin, and chymotrypsin on immobilized aprotinin
- Quantification of kallikrein activity in mixtures of esterases and proteases
- Controlled degradation of substrates by avoiding nonspecific proteolysis in clinical chemical tests
- Aprotinin as a model protein in protein-folding studies
- Molecular weight marker in SDS-polyacrylamide gel electrophoresis
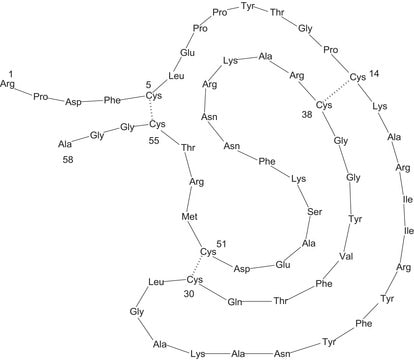
서열
Monomeric peptide of 58 amino acids held in conformation by three disulfide bonds.
단위 정의
One inhibitor unit (IU) is defined as the amount of aprotinin that completely inhibits 1 U trypsin in < 10 minutes at pH 6. (Trypsin activity determined at +25 °C, pH 8.0, BAEE as substrate).
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 2.8 inhibitor units (+25 °C, Chromozym TRY as substrate).
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 26 kallikrein inhibitor units (KIU) (+25 °C).
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 0.067 inhibitor units (+25 °C; Bz-D,L-Arg-4-Na as substrate, trypsin determination at pH 7.8).
One kallikrein inhibitor unit = 0.17 μg crystalline aprotinin.
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 2.8 inhibitor units (+25 °C, Chromozym TRY as substrate).
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 26 kallikrein inhibitor units (KIU) (+25 °C).
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 0.067 inhibitor units (+25 °C; Bz-D,L-Arg-4-Na as substrate, trypsin determination at pH 7.8).
One kallikrein inhibitor unit = 0.17 μg crystalline aprotinin.
제조 메모
Working concentration: 0.06 to 2 μg/ml (0.01 - 0.3 μM)
Working solution: Soluble in water (10 mg/ml) or aqueous buffer solution (e.g., 0.1 M Tris, pH 8.0).
Note: To avoid adsorption of aprotinin onto negatively charged solid phases, e.g., chromatography gels, ultrafiltration membranes, the NaCl concentration should be above 0.1 M or other suitable salts should be added to all buffers used during the separation.
Storage conditions (working solution): -15 to -25 °C
Working solution: Soluble in water (10 mg/ml) or aqueous buffer solution (e.g., 0.1 M Tris, pH 8.0).
Note: To avoid adsorption of aprotinin onto negatively charged solid phases, e.g., chromatography gels, ultrafiltration membranes, the NaCl concentration should be above 0.1 M or other suitable salts should be added to all buffers used during the separation.
Storage conditions (working solution): -15 to -25 °C
재구성
Freely soluble in water (10 mg/ml) or aqueous buffer solution (e.g., Tris, 0.1 M, pH 8.0). A solution adjusted to pH 7 to 8 is stable for approximately 1 week at 2 to 8 °C.
Aliquots stored at -15 to -25 °C are stable for approximately 6 months.
Note: Avoid repeated freezing and thawing and exposure to strongly alkaline solutions (inactive at pH > 12.8).
Aliquots stored at -15 to -25 °C are stable for approximately 6 months.
Note: Avoid repeated freezing and thawing and exposure to strongly alkaline solutions (inactive at pH > 12.8).
기타 정보
For life science research only. Not for use in diagnostic procedures.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Kamlesh Shroff et al.
Langmuir : the ACS journal of surfaces and colloids, 28(3), 1858-1865 (2011-12-14)
In recent years, a variety of biomimetic constructs have emerged which mimic the bioactive sequences found in the natural extracellular matrix (ECM) proteins such as fibronectin (FN) that promote cell adhesion as well as proliferation on artificially functionalized interfaces. Much
[EXPERIMENTS ON THE ISOLATION OF THE KALLIKREIN INACTIVATOR. V. THE ISOLATION OF A KALLIKREIN INACTIVATOR FROM THE BOVINE LUNG AND ITS IDENTIFICATION WITH THE INHIBITOR FROM THE BOVINE PAROTID GLAND].
H KRAUT et al.
Hoppe-Seyler's Zeitschrift fur physiologische Chemie, 338, 231-237 (1964-01-01)
H Fritz et al.
Hoppe-Seyler's Zeitschrift fur physiologische Chemie, 360(3), 437-444 (1979-03-01)
Using the indirect immunofluorescence technique, the basic kallikrein-trypsin inhibitor of bovine organs, Trasylol, could be localized in tissue mast cells of bovine lung, liver, pancreas and parotid gland. Identification of cells exhibiting specific fluorescence as tissue mast cells was achieved
Glucose Starvation Increases V-ATPase Assembly and Activity in Mammalian Cells through AMP
Kinase and Phosphatidylinositide 3-Kinase/Akt Signaling
Kinase and Phosphatidylinositide 3-Kinase/Akt Signaling
Christina M. McGuire and Michael Forgac
The Journal of Biological Chemistry (2018)
Selecting protein N-terminal peptides by combined
fractional diagonal chromatography
fractional diagonal chromatography
An S, et al.
Nature Protocols (2011)
문서
While aprotinin and bovine pancreatic trypsin inhibitor (BPTI) are the same protein sequence, the term aprotinin is typically used when describing the protein derived from bovine lung.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.