모든 사진(4)
About This Item
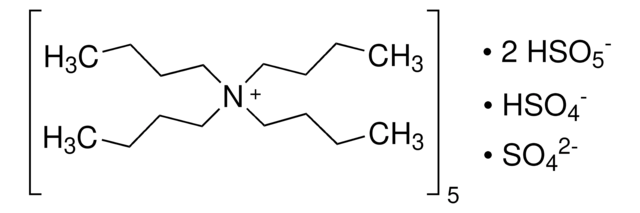
Linear Formula:
KHSO5 · 0.5KHSO4 · 0.5K2SO4
CAS Number:
Molecular Weight:
307.38
MDL number:
UNSPSC 코드:
12352300
PubChem Substance ID:
NACRES:
NA.21
추천 제품
vapor pressure
<0.0000017 hPa
Quality Level
형태
powder
반응 적합성
reagent type: oxidant
농도
>4.0% (active oxygen basis (by Na2S2O3, titration))
pH
2.1 (77 °C, 30 g/L)
SMILES string
[K+].[K+].[K+].[K+].[K+].OS([O-])(=O)=O.[O-]S([O-])(=O)=O.O[S+]([O-])([O-])(=O)=O.O[S+]([O-])([O-])(=O)=O
InChI
1S/5K.2H2O5S.2H2O4S/c;;;;;2*1-5-6(2,3)4;2*1-5(2,3)4/h;;;;;2*1H,(H,2,3,4);2*(H2,1,2,3,4)/q5*+1;;;;/p-5
InChI key
HJKYXKSLRZKNSI-UHFFFAOYSA-I
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
OXONE®, monopersulfate compound is a potassium triple salt mainly used as a stable, easy to handle and nontoxic oxidant.
애플리케이션
A rapid, efficient synthesis of oxaziridines from imines using buffered OXONE has been reported. Also used to study fading of an artist′s colorants.
OXONE®, monopersulfate compound may be used as an alternative to transition-metal oxidants for the conversion of aldehydes to carboxylic acids or esters.
Oxidant used for halogenation of α,β-unsaturated carbonyl compounds and catalytic generation of hypervalent iodine reagents for alcohol oxidation.
Oxidant used for halogenation of a,b-unsaturated carbonyl compounds and catalytic generation of hypervalent iodine reagents for alcohol oxidation.
2-Iodoxybenzenesulfonic Acid as an Extremely Active Catalyst for the Selective Oxidation of Alcohols to Aldehydes, Ketones, Carboxylic Acids, and Enones with Oxone
A Convenient Halogenation of α,β-Unsaturated Carbonyl Compounds with OXONE® and Hydrohalic Acid (HBr, HCl)
2-Iodoxybenzenesulfonic Acid as an Extremely Active Catalyst for the Selective Oxidation of Alcohols to Aldehydes, Ketones, Carboxylic Acids, and Enones with Oxone
A Convenient Halogenation of α,β-Unsaturated Carbonyl Compounds with OXONE® and Hydrohalic Acid (HBr, HCl)
법적 정보
OXONE is a registered trademark of E. I. du Pont de Nemours and Company
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Atmos. Environ., Part A, 27A, 765-765 (1993)
J Rodríguez-Chueca et al.
Journal of hazardous materials, 372, 94-102 (2018-05-08)
This study explores the enhancement of UV-C tertiary treatment by sulfate radical based Advanced Oxidation Processes (SR-AOPs), including photolytic activation of peroxymonosulfate (PMS) and persulfate (PS) and their photocatalytic activation using Fe(II). Their efficiency was assessed both for the inactivation
Yifu Li et al.
Bioresource technology, 262, 294-301 (2018-05-08)
In this study, zero valent iron (ZVI) activated peroxymonosulfate (PMS) as novel technique (i.e. ZVI-PMS technology) was employed to enhance sludge dewatering. In optimal sludge dewatering conditions of ZVI and KHSO5 dosages, the specific resistance to filtration (SRF) was reduced
Jiamin Hu et al.
Water science and technology : a journal of the International Association on Water Pollution Research, 79(5), 911-920 (2019-04-27)
In this study, the difference in oxidative capacity for removing antibiotics and the mechanism between the Cu(II)/peroxymonosulfate (PMS)/UV and Cu(II)/persulfate (PDS)/UV systems were compared under various conditions. The optimal Cu(II) concentration in the Cu(II)/PMS/UV system was 30 μM, and in
Journal of chemical research. Synopses, 388-388 (1992)
문서
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.